Vaccination is the mainstream measure against coronavirus disease 2019 (COVID-19). As of May 2022, more than 80% of the Japanese population has received vaccination doses (1), and booster vaccination programs are in progress. Acute disseminated encephalomyelitis is the most common type of demyelination following vaccinations, and although an increase in neuromyelitis optica spectrum disease (NMOSD) has recently been reported worldwide (2-4), no collective data, especially after newly developed mRNA vaccines against COVID-19, have been reported yet.
In Japan, mRNA vaccines, manufactured by Pfizer and Moderna, and ChAdOx1 vaccine, manufactured by AstraZeneca, have been used so far. To characterize optic neuritis after COVID-19 vaccination in Japan, we summarized information on such patients spontaneously reported by medical institutions throughout the country from February 17, 2021, to March 20, 2022, from the public disclosure website of the Ministry of Health, Labour and Welfare (5). Statistical analysis was not performed due to the small number of subjects. Ethical considerations were not applied to this study as we used only publicly available data.
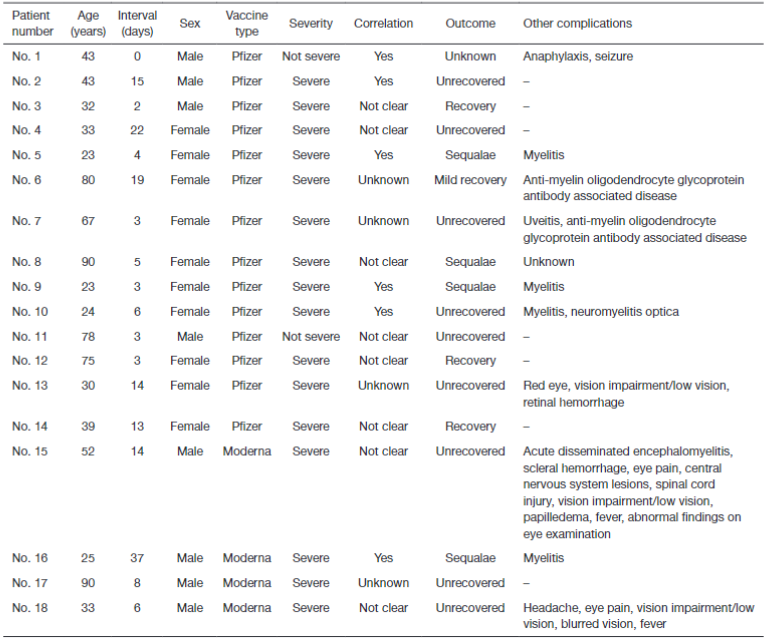
Table 1 summarizes the patient characteristics, and we identified 18 cases in total. All cases were after mRNA vaccinations; 14 (78%) were Pfizer, and 4 (22%) were Moderna. There were 10 (56%) female cases (median age 36 years; range, 23–90 years) and 8 (44%) male cases (median age 43 years; range, 25–90 years). The median interval from vaccination to disease onset was 5.5 days (range, 3–22 days) for females and 7 days (range, 0–37 days) for males. Severity was reported as severe or not severe, correlation was reported as yes or not clear, and outcome was reported as unrecovered, recovered, mildly recovered, with sequelae, or unknown. If there were complications, they were also reported, but these were not necessarily required to be reported, and in some cases they were not reported. Of the total, 16 (89%) were severe cases, and 4 (22%) were suffered from sequelae. As of March 20, 2022, Japan’s estimated Pfizer and Moderna vaccine doses numbered 196,160,191 and 51,618,647, respectively. Therefore, the incidence of optic neuritis after each vaccine is estimated to be 0.71 and 0.77 people per 10 million doses, respectively.
In this secondary analysis of the publicly available data, the incidence of optic neuritis was less than one case per 10 million doses for both Pfizer and Moderna vaccines. Basically, reported risk of optic neuritis in the Japanese population is 2 per 100,000. However, we could not find any previous studies on the incidence of optic neuritis after mRNA vaccination. Thus, we searched other post-vaccination optic neuritis and found that among the vaccine products used in Japan, inactivated influenza vaccines, recombinant hepatitis B vaccines, and inactivated Japanese encephalitis vaccines have been reported to be associated with acute disseminated encephalomyelitis causing central nervous system or optic neuritis complications at a frequency of 1 to 3.5 people per 10 million doses of vaccines (6). Therefore, while there may be biases such as underreported mild cases, the incidence of optic neuritis was less frequent with the COVID-19 mRNA vaccines than with other conventional vaccines. On the other hand, 10 of 18 patients (56%) developed optic neuritis within 1 week of vaccination. In acute disseminated encephalomyelitis, 55% of patients were also reported to have developed the disease within 1 week of vaccination (5). Although there may be a reporting bias concern, the distributions of days from vaccination to disease onset are not likely to be random distribution. A causal relationship to demyelinating disease from COVID-19 vaccination seems to exist, making it essential to pay close attention.
Of the 18 patients diagnosed with optic neuritis after COVID-19 vaccination, 16 (89%) were severe cases, suggesting this to be rare but a severe adverse reaction. Of note, at least two of these cases were positive for anti-myelin oligodendrocyte glycoprotein (MOG) antibodies. Anti-MOG antibodies are specific autoantibodies that cause inflammatory demyelinating lesions in the central nervous system, and the most common demyelinating events of increased anti-MOG antibodies have been reported with mRNA vaccines (7). A case of anti-MOG antibody-positive optic neuritis has also been reported from Mexico (3), and this diagnostic test is important because anti-MOG antibody-positive optic neuritis is a highly active disease with frequent recurrences and a high risk of blindness (8). In addition, since brainstem and spinal cord lesions may result after the development of optic neuritis (8), it is vital to identify anti-MOG antibody-positive optic neuritis and provide appropriate treatment in collaboration with neurologists and ophthalmologists.
This study has several limitations. Reports of adverse reactions after COVID-19 vaccination in Japan are based only on doctors’ and medical institutions’ submissions, with no standardized testing or diagnostic criteria, thus the number of reports may have been underestimated and we need to be careful about the interpretation. Similarly, regarding anti-MOG antibodies, the prevalence of testing was not available, and indeed more patients may have tested positive for such antibodies. The diagnostic accuracy and test criteria also need to be fully verified, thus the further studies are necessary to confirm the reports and to investigate details such as underlying diseases of the patients.
In conclusion, despite various biases, optic neuritis after COVID-19 vaccination with mRNA vaccines was a rare but serious adverse reaction. Meanwhile, the incidence was less frequent than in other vaccination programs in Japan. Hence, we should be careful with their interpretation.
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-22-45/coif). AO receives consulting fees from MNES Inc., outside the submitted work. He receives grant for his work on financial relationships between healthcare industry and healthcare professionals from Daiwa Japan Angro-Japan Foundation. He serves as a committee member for Japan Society for Medical Education. TT receives consulting fees from MNES Inc., and Bionics Co. Ltd., outside the submitted work. He receives grant for his work on financial relationships between healthcare industry and healthcare professionals from Daiwa Japan Angro-Japan Foundation. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.