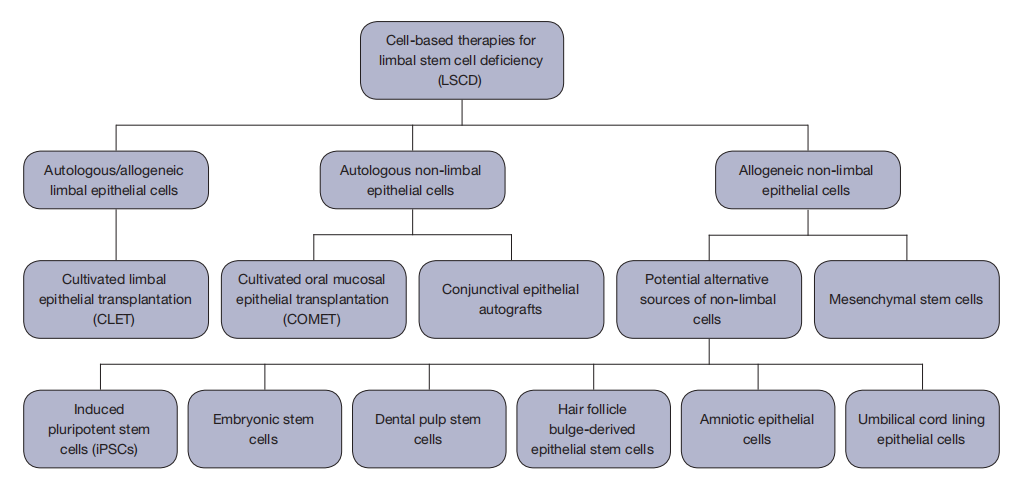
1、Elhusseiny AM, Soleimani M, Eleiwa TK, et al. Current
and Emerging Therapies for Limbal Stem Cell Deficiency.
Stem Cells Transl Med 2022;11:259-68.Elhusseiny AM, Soleimani M, Eleiwa TK, et al. Current
and Emerging Therapies for Limbal Stem Cell Deficiency.
Stem Cells Transl Med 2022;11:259-68.
2、Le Q, Chauhan T, Cordova D, et al. Biomarkers of in vivo
limbal stem cell function. Ocul Surf 2022;23:123-30.Le Q, Chauhan T, Cordova D, et al. Biomarkers of in vivo
limbal stem cell function. Ocul Surf 2022;23:123-30.
3、Schl%C3%B6tzer-Schrehardt%20U%2C%20Latta%20L%2C%20Gie%C3%9Fl%20A%2C%20et%20al.%20%0ADysfunction%20of%20the%20limbal%20epithelial%20stem%20cell%20niche%20in%20%0Aaniridia-associated%20keratopathy.%20Ocul%20Surf%202021%3B21%3A160-73.Schl%C3%B6tzer-Schrehardt%20U%2C%20Latta%20L%2C%20Gie%C3%9Fl%20A%2C%20et%20al.%20%0ADysfunction%20of%20the%20limbal%20epithelial%20stem%20cell%20niche%20in%20%0Aaniridia-associated%20keratopathy.%20Ocul%20Surf%202021%3B21%3A160-73.
4、Figueiredo FC, Glanville JM, Arber M, et al. A systematic
review of cellular therapies for the treatment of limbal
stem cell deficiency affecting one or both eyes. Ocul Surf
2021;20:48-61.Figueiredo FC, Glanville JM, Arber M, et al. A systematic
review of cellular therapies for the treatment of limbal
stem cell deficiency affecting one or both eyes. Ocul Surf
2021;20:48-61.
5、Pellegrini G, Traverso CE, Franzi AT, et al. Long-term
restoration of damaged corneal surfaces with autologous
cultivated corneal epithelium. Lancet 1997;349:990-3.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term
restoration of damaged corneal surfaces with autologous
cultivated corneal epithelium. Lancet 1997;349:990-3.
6、Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell
therapy and long-term corneal regeneration. N Engl J
Med 2010;363:147-55.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell
therapy and long-term corneal regeneration. N Engl J
Med 2010;363:147-55.
7、Sangwan VS, Basu S, Vemuganti GK, et al. Clinical
outcomes of xeno-free autologous cultivated limbal
epithelial transplantation: a 10-year study. Br J Ophthalmol
2011;95:1525-9.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical
outcomes of xeno-free autologous cultivated limbal
epithelial transplantation: a 10-year study. Br J Ophthalmol
2011;95:1525-9.
8、Fasolo A, Pedrotti E, Passilongo M, et al. Safety outcomes
and long-term effectiveness of ex vivo autologous cultured
limbal epithelial transplantation for limbal stem cell
deficiency. Br J Ophthalmol 2017;101:640-9.Fasolo A, Pedrotti E, Passilongo M, et al. Safety outcomes
and long-term effectiveness of ex vivo autologous cultured
limbal epithelial transplantation for limbal stem cell
deficiency. Br J Ophthalmol 2017;101:640-9.
9、Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat
autologous cultivated limbal epithelial transplantation for
ocular surface burns. Am J Ophthalmol 2012;153:643-50,
650.e1-2.Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat
autologous cultivated limbal epithelial transplantation for
ocular surface burns. Am J Ophthalmol 2012;153:643-50,
650.e1-2.
10、Cheung AY, Sarnicola E, Holland EJ. Long-Term Ocular
Surface Stability in Conjunctival Limbal Autograft Donor
Eyes. Cornea 2017;36:1031-5.Cheung AY, Sarnicola E, Holland EJ. Long-Term Ocular
Surface Stability in Conjunctival Limbal Autograft Donor
Eyes. Cornea 2017;36:1031-5.
11、Sharma R, Sharma A, Nirankari VS. Cultivated Limbal
Epithelial Transplant Versus Conjunctival Limbal Auto
Transplant in Uniocular Limbal Stem Cell Deficiency:
Long-Term Results. Medical Research Archives 2022;10.
doi: 10.18103/mra.v10i7.2956.Sharma R, Sharma A, Nirankari VS. Cultivated Limbal
Epithelial Transplant Versus Conjunctival Limbal Auto
Transplant in Uniocular Limbal Stem Cell Deficiency:
Long-Term Results. Medical Research Archives 2022;10.
doi: 10.18103/mra.v10i7.2956.
12、Sejpal K, Ali MH, Maddileti S, et al. Cultivated limbal
epithelial transplantation in children with ocular surface
burns. JAMA Ophthalmol 2013;131:731-6.Sejpal K, Ali MH, Maddileti S, et al. Cultivated limbal
epithelial transplantation in children with ocular surface
burns. JAMA Ophthalmol 2013;131:731-6.
13、Mishan MA, Yaseri M, Baradaran-Rafii A, et al.
Systematic review and meta-analysis investigating
autograft versus allograft cultivated limbal epithelial
transplantation in limbal stem cell deficiency. Int
Ophthalmol 2019;39:2685-96.Mishan MA, Yaseri M, Baradaran-Rafii A, et al.
Systematic review and meta-analysis investigating
autograft versus allograft cultivated limbal epithelial
transplantation in limbal stem cell deficiency. Int
Ophthalmol 2019;39:2685-96.
14、Pellegrini G, Ardigò D, Milazzo G, et al. Navigating
Market Authorization: The Path Holoclar Took to Become
the First Stem Cell Product Approved in the European
Union. Stem Cells Transl Med 2018;7:146-54.Pellegrini G, Ardigò D, Milazzo G, et al. Navigating
Market Authorization: The Path Holoclar Took to Become
the First Stem Cell Product Approved in the European
Union. Stem Cells Transl Med 2018;7:146-54.
15、Nakamura T, Inatomi T, Sotozono C, et al.
Transplantation of cultivated autologous oral mucosal
epithelial cells in patients with severe ocular surface
disorders. Br J Ophthalmol 2004;88:1280-4.Nakamura T, Inatomi T, Sotozono C, et al.
Transplantation of cultivated autologous oral mucosal
epithelial cells in patients with severe ocular surface
disorders. Br J Ophthalmol 2004;88:1280-4.
16、Satake Y, Higa K, Tsubota K, et al. Long-term outcome
of cultivated oral mucosal epithelial sheet transplantation
in treatment of total limbal stem cell deficiency.
Ophthalmology 2011;118:1524-30.Satake Y, Higa K, Tsubota K, et al. Long-term outcome
of cultivated oral mucosal epithelial sheet transplantation
in treatment of total limbal stem cell deficiency.
Ophthalmology 2011;118:1524-30.
17、Satake Y, Yamaguchi T, Hirayama M, et al. Ocular
surface reconstruction by cultivated epithelial sheet
transplantation. Cornea 2014;33 Suppl 11:S42-6.Satake Y, Yamaguchi T, Hirayama M, et al. Ocular
surface reconstruction by cultivated epithelial sheet
transplantation. Cornea 2014;33 Suppl 11:S42-6.
18、Sotozono C, Inatomi T, Nakamura T, et al. Visual
improvement after cultivated oral mucosal epithelial
transplantation. Ophthalmology 2013;120:193-200.Sotozono C, Inatomi T, Nakamura T, et al. Visual
improvement after cultivated oral mucosal epithelial
transplantation. Ophthalmology 2013;120:193-200.
19、Komai S, Inatomi T, Nakamura T, et al. Long-term outcome of cultivated oral mucosal epithelial
transplantation for fornix reconstruction in chronic
cicatrising diseases. Br J Ophthalmol 2022;106:1355-62.Komai S, Inatomi T, Nakamura T, et al. Long-term outcome of cultivated oral mucosal epithelial
transplantation for fornix reconstruction in chronic
cicatrising diseases. Br J Ophthalmol 2022;106:1355-62.
20、Cabral JV, Jackson CJ, Utheim TP, et al. Ex vivo
cultivated oral mucosal epithelial cell transplantation for
limbal stem cell deficiency: a review. Stem Cell Res Ther
2020;11:301.Cabral JV, Jackson CJ, Utheim TP, et al. Ex vivo
cultivated oral mucosal epithelial cell transplantation for
limbal stem cell deficiency: a review. Stem Cell Res Ther
2020;11:301.
21、Dobrowolski D, Orzechowska-Wylegala B, Wowra B, et
al. Cultivated Oral Mucosa Epithelium in Ocular Surface
Reconstruction in Aniridia Patients. Biomed Res Int
2015;2015:281870.Dobrowolski D, Orzechowska-Wylegala B, Wowra B, et
al. Cultivated Oral Mucosa Epithelium in Ocular Surface
Reconstruction in Aniridia Patients. Biomed Res Int
2015;2015:281870.
22、Venugopal R, Nagpal R, Mohanty S, et al. Outcomes of
Cultivated Oral Mucosal Epithelial Transplantation in
Eyes With Chronic Stevens-Johnson Syndrome Sequelae.
Am J Ophthalmol 2021;222:82-91.Venugopal R, Nagpal R, Mohanty S, et al. Outcomes of
Cultivated Oral Mucosal Epithelial Transplantation in
Eyes With Chronic Stevens-Johnson Syndrome Sequelae.
Am J Ophthalmol 2021;222:82-91.
23、Wang J, Qi X, Dong Y, et al. Comparison of the efficacy
of different cell sources for transplantation in total limbal
stem cell deficiency. Graefes Arch Clin Exp Ophthalmol
2019;257:1253-63.Wang J, Qi X, Dong Y, et al. Comparison of the efficacy
of different cell sources for transplantation in total limbal
stem cell deficiency. Graefes Arch Clin Exp Ophthalmol
2019;257:1253-63.
24、Prabhasawat P, Chirapapaisan C, Jiravarnsirikul A, et
al. Phenotypic Characterization of Corneal Epithelium
in Long-Term Follow-Up of Patients Post-Autologous
Cultivated Oral Mucosal Epithelial Transplantation.
Cornea 2021;40:842-50.Prabhasawat P, Chirapapaisan C, Jiravarnsirikul A, et
al. Phenotypic Characterization of Corneal Epithelium
in Long-Term Follow-Up of Patients Post-Autologous
Cultivated Oral Mucosal Epithelial Transplantation.
Cornea 2021;40:842-50.
25、Ang LP, Tanioka H, Kawasaki S, et al. Cultivated human
conjunctival epithelial transplantation for total limbal stem
cell deficiency. Invest Ophthalmol Vis Sci 2010;51:758-64.Ang LP, Tanioka H, Kawasaki S, et al. Cultivated human
conjunctival epithelial transplantation for total limbal stem
cell deficiency. Invest Ophthalmol Vis Sci 2010;51:758-64.
26、Jeon S, Choi SH, Wolosin JM, et al. Regeneration of the
corneal epithelium with conjunctival epithelial equivalents
generated in serum- and feeder-cell-free media. Mol Vis
2013;19:2542-50.Jeon S, Choi SH, Wolosin JM, et al. Regeneration of the
corneal epithelium with conjunctival epithelial equivalents
generated in serum- and feeder-cell-free media. Mol Vis
2013;19:2542-50.
27、Silber PC, Ricardo JR, Cristovam PC, et al. Conjunctival
epithelial cells cultivated ex vivo from patients with total
limbal stem cell deficiency. Eur J Ophthalmol 2014. [Epub
ahead of print]. doi: 10.5301/ejo.5000511.Silber PC, Ricardo JR, Cristovam PC, et al. Conjunctival
epithelial cells cultivated ex vivo from patients with total
limbal stem cell deficiency. Eur J Ophthalmol 2014. [Epub
ahead of print]. doi: 10.5301/ejo.5000511.
28、Ricardo JR, Cristovam PC, Filho PA, et al.
Transplantation of conjunctival epithelial cells cultivated
ex vivo in patients with total limbal stem cell deficiency.
Cornea 2013;32:221-8.Ricardo JR, Cristovam PC, Filho PA, et al.
Transplantation of conjunctival epithelial cells cultivated
ex vivo in patients with total limbal stem cell deficiency.
Cornea 2013;32:221-8.
29、Casaroli-Marano RP, Nieto-Nicolau N, Martínez-Conesa
EM, et al. Potential Role of Induced Pluripotent Stem
Cells (IPSCs) for Cell-Based Therapy of the Ocular
Surface. J Clin Med 2015;4:318-42.Casaroli-Marano RP, Nieto-Nicolau N, Martínez-Conesa
EM, et al. Potential Role of Induced Pluripotent Stem
Cells (IPSCs) for Cell-Based Therapy of the Ocular
Surface. J Clin Med 2015;4:318-42.
30、Zhu J, Slevin M, Guo BQ, et al. Induced pluripotent stem
cells as a potential therapeutic source for corneal epithelial
stem cells. Int J Ophthalmol 2018;11:2004-10.Zhu J, Slevin M, Guo BQ, et al. Induced pluripotent stem
cells as a potential therapeutic source for corneal epithelial
stem cells. Int J Ophthalmol 2018;11:2004-10.
31、Notara M, Hernandez D, Mason C, et al. Characterization
of the phenotype and functionality of corneal epithelial
cells derived from mouse embryonic stem cells. Regen
Med 2012;7:167-78.Notara M, Hernandez D, Mason C, et al. Characterization
of the phenotype and functionality of corneal epithelial
cells derived from mouse embryonic stem cells. Regen
Med 2012;7:167-78.
32、Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal
epithelial cells from induced pluripotent stem cells
derived from human dermal fibroblast and corneal limbal
epithelium. PLoS One 2012;7:e45435.Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal
epithelial cells from induced pluripotent stem cells
derived from human dermal fibroblast and corneal limbal
epithelium. PLoS One 2012;7:e45435.
33、Sareen D, Saghizadeh M, Ornelas L, et al. Differentiation
of human limbal-derived induced pluripotent stem cells
into limbal-like epithelium. Stem Cells Transl Med
2014;3:1002-12.Sareen D, Saghizadeh M, Ornelas L, et al. Differentiation
of human limbal-derived induced pluripotent stem cells
into limbal-like epithelium. Stem Cells Transl Med
2014;3:1002-12.
34、Mikhailova A, Ilmarinen T, Ratnayake A, et al. Human
pluripotent stem cell-derived limbal epithelial stem cells
on bioengineered matrices for corneal reconstruction. Exp
Eye Res 2016;146:26-34.Mikhailova A, Ilmarinen T, Ratnayake A, et al. Human
pluripotent stem cell-derived limbal epithelial stem cells
on bioengineered matrices for corneal reconstruction. Exp
Eye Res 2016;146:26-34.
35、Hayashi R, Ishikawa Y, Sasamoto Y, et al. Co-ordinated
ocular development from human iPS cells and recovery of
corneal function. Nature 2016;531:376-80.Hayashi R, Ishikawa Y, Sasamoto Y, et al. Co-ordinated
ocular development from human iPS cells and recovery of
corneal function. Nature 2016;531:376-80.
36、Hayashi R, Ishikawa Y, Katori R, et al. Coordinated
generation of multiple ocular-like cell lineages and
fabrication of functional corneal epithelial cell sheets from
human iPS cells. Nat Protoc 2017;12:683-96.Hayashi R, Ishikawa Y, Katori R, et al. Coordinated
generation of multiple ocular-like cell lineages and
fabrication of functional corneal epithelial cell sheets from
human iPS cells. Nat Protoc 2017;12:683-96.
37、Hongisto H, Vattulainen M, Ilmarinen T, et al. Efficient
and Scalable Directed Differentiation of Clinically
Compatible Corneal Limbal Epithelial Stem Cells from
Human Pluripotent Stem Cells. J Vis Exp 2018;58279.Hongisto H, Vattulainen M, Ilmarinen T, et al. Efficient
and Scalable Directed Differentiation of Clinically
Compatible Corneal Limbal Epithelial Stem Cells from
Human Pluripotent Stem Cells. J Vis Exp 2018;58279.
38、Vattulainen%20M%2C%20Ilmarinen%20T%2C%20Viheri%C3%A4l%C3%A4%20T%2C%20et%20al.%20Corneal%20%0Aepithelial%20differentiation%20of%20human%20pluripotent%20stem%20cells%20%0Agenerates%20ABCB5(%2B)%20and%20%E2%88%86Np63%CE%B1(%2B)%20cells%20with%20limbal%20cell%20%0Acharacteristics%20and%20high%20wound%20healing%20capacity.%20Stem%20Cell%20%0ARes%20Ther%202021%3B12%3A609.Vattulainen%20M%2C%20Ilmarinen%20T%2C%20Viheri%C3%A4l%C3%A4%20T%2C%20et%20al.%20Corneal%20%0Aepithelial%20differentiation%20of%20human%20pluripotent%20stem%20cells%20%0Agenerates%20ABCB5(%2B)%20and%20%E2%88%86Np63%CE%B1(%2B)%20cells%20with%20limbal%20cell%20%0Acharacteristics%20and%20high%20wound%20healing%20capacity.%20Stem%20Cell%20%0ARes%20Ther%202021%3B12%3A609.
39、Reza HM, Ng BY, Gimeno FL, et al. Umbilical cord
lining stem cells as a novel and promising source for ocular
surface regeneration. Stem Cell Rev Rep 2011;7:935-47.Reza HM, Ng BY, Gimeno FL, et al. Umbilical cord
lining stem cells as a novel and promising source for ocular
surface regeneration. Stem Cell Rev Rep 2011;7:935-47.
40、Ang LP, Jain P, Phan TT, et al. Human Umbilical
Cord Lining Cells as Novel Feeder Layer for Ex Vivo
Cultivation of Limbal Epithelial Cells. Invest Ophthalmol
Vis Sci 2015;56:4697-704.Ang LP, Jain P, Phan TT, et al. Human Umbilical
Cord Lining Cells as Novel Feeder Layer for Ex Vivo
Cultivation of Limbal Epithelial Cells. Invest Ophthalmol
Vis Sci 2015;56:4697-704.
41、Meyer-Blazejewska EA, Call MK, Yamanaka O, et al.
From hair to cornea: toward the therapeutic use of hair
follicle-derived stem cells in the treatment of limbal stem
cell deficiency. Stem Cells 2011;29:57-66.Meyer-Blazejewska EA, Call MK, Yamanaka O, et al.
From hair to cornea: toward the therapeutic use of hair
follicle-derived stem cells in the treatment of limbal stem
cell deficiency. Stem Cells 2011;29:57-66.
42、Call M, Meyer EA, Kao WW, et al. Murine Hair Follicle
Derived Stem Cell Transplantation onto the Cornea Using
a Fibrin Carrier. Bio Protoc 2018;8:e2849.Call M, Meyer EA, Kao WW, et al. Murine Hair Follicle
Derived Stem Cell Transplantation onto the Cornea Using
a Fibrin Carrier. Bio Protoc 2018;8:e2849.
43、Monteiro BG, Serafim RC, Melo GB, et al. Human
immature dental pulp stem cells share key characteristic
features with limbal stem cells. Cell Prolif 2009;42:587-94.Monteiro BG, Serafim RC, Melo GB, et al. Human
immature dental pulp stem cells share key characteristic
features with limbal stem cells. Cell Prolif 2009;42:587-94.
44、Gomes JA, Geraldes Monteiro B, Melo GB, et al.
Corneal reconstruction with tissue-engineered cell sheets
composed of human immature dental pulp stem cells.
Invest Ophthalmol Vis Sci 2010;51:1408-14.Gomes JA, Geraldes Monteiro B, Melo GB, et al.
Corneal reconstruction with tissue-engineered cell sheets
composed of human immature dental pulp stem cells.
Invest Ophthalmol Vis Sci 2010;51:1408-14.
45、Patil S, D'Souza C, Patil P, et al. Culture and
characterization of human dental pulp derived stem cells as
limbal stem cells for corneal damage repair. Mol Med Rep
2019;20:4688-94.Patil S, D'Souza C, Patil P, et al. Culture and
characterization of human dental pulp derived stem cells as
limbal stem cells for corneal damage repair. Mol Med Rep
2019;20:4688-94.
46、Monteiro BG, Loureiro RR, Cristovam PC, et al.
Amniotic membrane as a biological scaffold for dental pulp
stem cell transplantation in ocular surface reconstruction.
Arq Bras Oftalmol 2019;82:32-7.Monteiro BG, Loureiro RR, Cristovam PC, et al.
Amniotic membrane as a biological scaffold for dental pulp
stem cell transplantation in ocular surface reconstruction.
Arq Bras Oftalmol 2019;82:32-7.
47、Kim JH, Chun YS, Lee SH, et al. Ocular surface
reconstruction with autologous nasal mucosa in
cicatricial ocular surface disease. Am J Ophthalmol
2010;149:45-53.Kim JH, Chun YS, Lee SH, et al. Ocular surface
reconstruction with autologous nasal mucosa in
cicatricial ocular surface disease. Am J Ophthalmol
2010;149:45-53.
48、Kobayashi M, Nakamura T, Yasuda M, et al. Ocular
surface reconstruction with a tissue-engineered nasal
mucosal epithelial cell sheet for the treatment of
severe ocular surface diseases. Stem Cells Transl Med
2015;4:99-109.Kobayashi M, Nakamura T, Yasuda M, et al. Ocular
surface reconstruction with a tissue-engineered nasal
mucosal epithelial cell sheet for the treatment of
severe ocular surface diseases. Stem Cells Transl Med
2015;4:99-109.
49、Chen HC, Yeh LK, Tsai YJ, et al. Expression of
angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Invest
Ophthalmol Vis Sci 2012;53:5615-23.Chen HC, Yeh LK, Tsai YJ, et al. Expression of
angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Invest
Ophthalmol Vis Sci 2012;53:5615-23.
50、Kanayama S, Nishida K, Yamato M, et al. Analysis
of angiogenesis induced by cultured corneal and oral
mucosal epithelial cell sheets in vitro. Exp Eye Res
2007;85:772-81.Kanayama S, Nishida K, Yamato M, et al. Analysis
of angiogenesis induced by cultured corneal and oral
mucosal epithelial cell sheets in vitro. Exp Eye Res
2007;85:772-81.
51、Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria
for defining multipotent mesenchymal stromal cells.
The International Society for Cellular Therapy position
statement. Cytotherapy 2006;8:315-7.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria
for defining multipotent mesenchymal stromal cells.
The International Society for Cellular Therapy position
statement. Cytotherapy 2006;8:315-7.
52、Berebichez-Fridman R, Montero-Olvera PR. Sources
and Clinical Applications of Mesenchymal Stem Cells:
State-of-the-art review. Sultan Qaboos Univ Med J
2018;18:e264-77.Berebichez-Fridman R, Montero-Olvera PR. Sources
and Clinical Applications of Mesenchymal Stem Cells:
State-of-the-art review. Sultan Qaboos Univ Med J
2018;18:e264-77.
53、Liang L, Luo X, Zhang J, et al. Safety and feasibility of
subconjunctival injection of mesenchymal stem cells for
acute severe ocular burns: A single-arm study. Ocul Surf
2021;22:103-9.Liang L, Luo X, Zhang J, et al. Safety and feasibility of
subconjunctival injection of mesenchymal stem cells for
acute severe ocular burns: A single-arm study. Ocul Surf
2021;22:103-9.
54、Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically
burned rat corneal surface by bone marrow-derived human
mesenchymal stem cells. Stem Cells 2006;24:315-21.Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically
burned rat corneal surface by bone marrow-derived human
mesenchymal stem cells. Stem Cells 2006;24:315-21.
55、Lan Y, Kodati S, Lee HS, et al. Kinetics and function
of mesenchymal stem cells in corneal injury. Invest
Ophthalmol Vis Sci 2012;53:3638-44.Lan Y, Kodati S, Lee HS, et al. Kinetics and function
of mesenchymal stem cells in corneal injury. Invest
Ophthalmol Vis Sci 2012;53:3638-44.
56、Cejkova J, Trosan P, Cejka C, et al. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal
stem cells growing on nanofiber scaffolds and transferred
onto the damaged corneal surface. Exp Eye Res
2013;116:312-23.Cejkova J, Trosan P, Cejka C, et al. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal
stem cells growing on nanofiber scaffolds and transferred
onto the damaged corneal surface. Exp Eye Res
2013;116:312-23.
57、Espandar L, Caldwell D, Watson R, et al. Application of
adipose-derived stem cells on scleral contact lens carrier
in an animal model of severe acute alkaline burn. Eye
Contact Lens 2014;40:243-7.Espandar L, Caldwell D, Watson R, et al. Application of
adipose-derived stem cells on scleral contact lens carrier
in an animal model of severe acute alkaline burn. Eye
Contact Lens 2014;40:243-7.
58、Bu P, Vin AP, Sethupathi P, et al. Effects of activated
omental cells on rat limbal corneal alkali injury. Exp Eye
Res 2014;121:143-6.Bu P, Vin AP, Sethupathi P, et al. Effects of activated
omental cells on rat limbal corneal alkali injury. Exp Eye
Res 2014;121:143-6.
59、Acar U, Pinarli FA, Acar DE, et al. Effect of allogeneic
limbal mesenchymal stem cell therapy in corneal
healing: role of administration route. Ophthalmic Res
2015;53:82-9.Acar U, Pinarli FA, Acar DE, et al. Effect of allogeneic
limbal mesenchymal stem cell therapy in corneal
healing: role of administration route. Ophthalmic Res
2015;53:82-9.
60、Ke Y, Wu Y, Cui X, et al. Polysaccharide hydrogel
combined with mesenchymal stem cells promotes
the healing of corneal alkali burn in rats. PLoS One
2015;10:e0119725.Ke Y, Wu Y, Cui X, et al. Polysaccharide hydrogel
combined with mesenchymal stem cells promotes
the healing of corneal alkali burn in rats. PLoS One
2015;10:e0119725.
61、Holan V, Trosan P, Cejka C, et al. A Comparative Study
of the Therapeutic Potential of Mesenchymal Stem Cells
and Limbal Epithelial Stem Cells for Ocular Surface
Reconstruction. Stem Cells Transl Med 2015;4:1052-63.Holan V, Trosan P, Cejka C, et al. A Comparative Study
of the Therapeutic Potential of Mesenchymal Stem Cells
and Limbal Epithelial Stem Cells for Ocular Surface
Reconstruction. Stem Cells Transl Med 2015;4:1052-63.
62、Lee MJ, Ko AY, Ko JH, et al. Mesenchymal stem/
stromal cells protect the ocular surface by suppressing
inflammation in an experimental dry eye. Mol Ther
2015;23:139-46.
Lee MJ, Ko AY, Ko JH, et al. Mesenchymal stem/
stromal cells protect the ocular surface by suppressing
inflammation in an experimental dry eye. Mol Ther
2015;23:139-46.
63、Cejka C, Cejkova J, Trosan P, et al. Transfer of
mesenchymal stem cells and cyclosporine A on alkali-injured rabbit cornea using nanofiber scaffolds strongly
reduces corneal neovascularization and scar formation.
Histol Histopathol 2016;31:969-80.Cejka C, Cejkova J, Trosan P, et al. Transfer of
mesenchymal stem cells and cyclosporine A on alkali-injured rabbit cornea using nanofiber scaffolds strongly
reduces corneal neovascularization and scar formation.
Histol Histopathol 2016;31:969-80.
64、Li G, Zhang Y, Cai S, et al. Human limbal niche cells
are a powerful regenerative source for the prevention
of limbal stem cell deficiency in a rabbit model. Sci Rep
2018;8:6566.Li G, Zhang Y, Cai S, et al. Human limbal niche cells
are a powerful regenerative source for the prevention
of limbal stem cell deficiency in a rabbit model. Sci Rep
2018;8:6566.
65、Calonge M, Pérez I, Galindo S, et al. A proof-of-concept
clinical trial using mesenchymal stem cells for the
treatment of corneal epithelial stem cell deficiency. Transl
Res 2019;206:18-40.Calonge M, Pérez I, Galindo S, et al. A proof-of-concept
clinical trial using mesenchymal stem cells for the
treatment of corneal epithelial stem cell deficiency. Transl
Res 2019;206:18-40.
66、Zeppieri M, Salvetat ML, Beltrami AP, et al. Human
adipose-derived stem cells for the treatment of chemically
burned rat cornea: preliminary results. Curr Eye Res
2013;38:451-63.Zeppieri M, Salvetat ML, Beltrami AP, et al. Human
adipose-derived stem cells for the treatment of chemically
burned rat cornea: preliminary results. Curr Eye Res
2013;38:451-63.
67、Ye J, Yao K, Kim JC. Mesenchymal stem cell
transplantation in a rabbit corneal alkali burn model:
engraftment and involvement in wound healing. Eye
(Lond) 2006;20:482-90.Ye J, Yao K, Kim JC. Mesenchymal stem cell
transplantation in a rabbit corneal alkali burn model:
engraftment and involvement in wound healing. Eye
(Lond) 2006;20:482-90.
68、Mittal SK, Omoto M, Amouzegar A, et al. Restoration of
Corneal Transparency by Mesenchymal Stem Cells. Stem
Cell Reports 2016;7:583-90.Mittal SK, Omoto M, Amouzegar A, et al. Restoration of
Corneal Transparency by Mesenchymal Stem Cells. Stem
Cell Reports 2016;7:583-90.
69、Galindo S, de la Mata A, López-Paniagua M, et al.
Subconjunctival injection of mesenchymal stem cells for
corneal failure due to limbal stem cell deficiency: state of
the art. Stem Cell Res Ther 2021;12:60.Galindo S, de la Mata A, López-Paniagua M, et al.
Subconjunctival injection of mesenchymal stem cells for
corneal failure due to limbal stem cell deficiency: state of
the art. Stem Cell Res Ther 2021;12:60.
70、Cotsarelis G, Cheng SZ, Dong G, et al. Existence of
slow-cycling limbal epithelial basal cells that can be
preferentially stimulated to proliferate: implications on
epithelial stem cells. Cell 1989;57:201-9.Cotsarelis G, Cheng SZ, Dong G, et al. Existence of
slow-cycling limbal epithelial basal cells that can be
preferentially stimulated to proliferate: implications on
epithelial stem cells. Cell 1989;57:201-9.
71、Ahmed SK, Soliman AA, Omar SM, et al. Bone Marrow
Mesenchymal Stem Cell Transplantation in a Rabbit
Corneal Alkali Burn Model (A Histological and Immune
Histo-chemical Study). Int J Stem Cells 2015;8:69-78.Ahmed SK, Soliman AA, Omar SM, et al. Bone Marrow
Mesenchymal Stem Cell Transplantation in a Rabbit
Corneal Alkali Burn Model (A Histological and Immune
Histo-chemical Study). Int J Stem Cells 2015;8:69-78.
72、Reinshagen H, Auw-Haedrich C, Sorg RV, et al. Corneal
surface reconstruction using adult mesenchymal stem cells
in experimental limbal stem cell deficiency in rabbits. Acta
Ophthalmol 2011;89:741-8.Reinshagen H, Auw-Haedrich C, Sorg RV, et al. Corneal
surface reconstruction using adult mesenchymal stem cells
in experimental limbal stem cell deficiency in rabbits. Acta
Ophthalmol 2011;89:741-8.
73、Galindo S, Herreras JM, López-Paniagua M, et al.
Therapeutic Effect of Human Adipose Tissue-Derived
Mesenchymal Stem Cells in Experimental Corneal Failure
Due to Limbal Stem Cell Niche Damage. Stem Cells 2017;35:2160-74.Galindo S, Herreras JM, López-Paniagua M, et al.
Therapeutic Effect of Human Adipose Tissue-Derived
Mesenchymal Stem Cells in Experimental Corneal Failure
Due to Limbal Stem Cell Niche Damage. Stem Cells 2017;35:2160-74.
74、Zajicova A, Pokorna K, Lencova A, et al. Treatment of
ocular surface injuries by limbal and mesenchymal stem
cells growing on nanofiber scaffolds. Cell Transplant
2010;19:1281-90.Zajicova A, Pokorna K, Lencova A, et al. Treatment of
ocular surface injuries by limbal and mesenchymal stem
cells growing on nanofiber scaffolds. Cell Transplant
2010;19:1281-90.
75、Rengasamy M, Gupta PK, Kolkundkar U, et al. Preclinical
safety & toxicity evaluation of pooled, allogeneic human
bone marrow-derived mesenchymal stromal cells. Indian J
Med Res 2016;144: 852-64.Rengasamy M, Gupta PK, Kolkundkar U, et al. Preclinical
safety & toxicity evaluation of pooled, allogeneic human
bone marrow-derived mesenchymal stromal cells. Indian J
Med Res 2016;144: 852-64.
76、Guess AJ, Daneault B, Wang R, et al. Safety Profile of
Good Manufacturing Practice Manufactured Interferon
γ-Primed Mesenchymal Stem/Stromal Cells for Clinical
Trials. Stem Cells Transl Med 2017;6:1868-79.Guess AJ, Daneault B, Wang R, et al. Safety Profile of
Good Manufacturing Practice Manufactured Interferon
γ-Primed Mesenchymal Stem/Stromal Cells for Clinical
Trials. Stem Cells Transl Med 2017;6:1868-79.
77、Gramlich OW, Burand AJ, Brown AJ, et al. Cryopreserved
Mesenchymal Stromal Cells Maintain Potency in a Retinal
Ischemia/Reperfusion Injury Model: Toward an off-the-shelf Therapy. Sci Rep 2016;6:26463.Gramlich OW, Burand AJ, Brown AJ, et al. Cryopreserved
Mesenchymal Stromal Cells Maintain Potency in a Retinal
Ischemia/Reperfusion Injury Model: Toward an off-the-shelf Therapy. Sci Rep 2016;6:26463.
78、Tappenbeck%20N%2C%20Schr%C3%B6der%20HM%2C%20Niebergall-Roth%20E%2C%20et%20%0Aal.%20In%20vivo%20safety%20profile%20and%20biodistribution%20of%20GMP-manufactured%20human%20skin-derived%20ABCB5-positive%20%0Amesenchymal%20stromal%20cells%20for%20use%20in%20clinical%20trials.%20%0ACytotherapy%202019%3B21%3A546-60.Tappenbeck%20N%2C%20Schr%C3%B6der%20HM%2C%20Niebergall-Roth%20E%2C%20et%20%0Aal.%20In%20vivo%20safety%20profile%20and%20biodistribution%20of%20GMP-manufactured%20human%20skin-derived%20ABCB5-positive%20%0Amesenchymal%20stromal%20cells%20for%20use%20in%20clinical%20trials.%20%0ACytotherapy%202019%3B21%3A546-60.
79、Labrador Velandia S, Di Lauro S, Alonso-Alonso ML,
et al. Biocompatibility of intravitreal injection of human
mesenchymal stem cells in immunocompetent rabbits.
Graefes Arch Clin Exp Ophthalmol 2018;256:125-34.Labrador Velandia S, Di Lauro S, Alonso-Alonso ML,
et al. Biocompatibility of intravitreal injection of human
mesenchymal stem cells in immunocompetent rabbits.
Graefes Arch Clin Exp Ophthalmol 2018;256:125-34.
80、Available online: https://clinicaltrials.gov/ct2/show/
NCT04626583Available online: https://clinicaltrials.gov/ct2/show/
NCT04626583
81、Delic NC, Cai JR, Watson SL, et al. Evaluating the
clinical translational relevance of animal models for
limbal stem cell deficiency: A systematic review. Ocul Surf
2022;23:169-83.Delic NC, Cai JR, Watson SL, et al. Evaluating the
clinical translational relevance of animal models for
limbal stem cell deficiency: A systematic review. Ocul Surf
2022;23:169-83.
82、Yu D, Chen M, Sun X, et al. Differentiation of mouse
induced pluripotent stem cells into corneal epithelial-like
cells. Cell Biol Int 2013;37:87-94.Yu D, Chen M, Sun X, et al. Differentiation of mouse
induced pluripotent stem cells into corneal epithelial-like
cells. Cell Biol Int 2013;37:87-94.
83、Available online: https://english.kyodonews.net/
news/2022/04/c8af6b7913b2-japan-team-proves-ips-based-cornea-transplant-safe-in-world-1st-trial.htmlAvailable online: https://english.kyodonews.net/
news/2022/04/c8af6b7913b2-japan-team-proves-ips-based-cornea-transplant-safe-in-world-1st-trial.html
84、Taylor CJ, Peacock S, Chaudhry AN, et al. Generating an
iPSC bank for HLA-matched tissue transplantation based
on known donor and recipient HLA types. Cell Stem Cell
2012;11:147-52.Taylor CJ, Peacock S, Chaudhry AN, et al. Generating an
iPSC bank for HLA-matched tissue transplantation based
on known donor and recipient HLA types. Cell Stem Cell
2012;11:147-52.
85、de Rham C, Villard J. Potential and limitation of HLA-based banking of human pluripotent stem cells for cell
therapy. J Immunol Res 2014;2014:518135.de Rham C, Villard J. Potential and limitation of HLA-based banking of human pluripotent stem cells for cell
therapy. J Immunol Res 2014;2014:518135.
86、Sullivan S, Stacey GN, Akazawa C, et al. Quality control
guidelines for clinical-grade human induced pluripotent
stem cell lines. Regen Med 2018;13:859-66.Sullivan S, Stacey GN, Akazawa C, et al. Quality control
guidelines for clinical-grade human induced pluripotent
stem cell lines. Regen Med 2018;13:859-66.
87、Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al.
Embryonic stem cell lines derived from human blastocysts.
Science 1998;282:1145-7.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al.
Embryonic stem cell lines derived from human blastocysts.
Science 1998;282:1145-7.
88、Hanson%20C%2C%20Hardarson%20T%2C%20Ellerstr%C3%B6m%20C%2C%20et%20al.%20%0ATransplantation%20of%20human%20embryonic%20stem%20cells%20onto%20a%20%0Apartially%20wounded%20human%20cornea%20in%20vitro.%20Acta%20Ophthalmol%20%0A2013%3B91%3A127-30.Hanson%20C%2C%20Hardarson%20T%2C%20Ellerstr%C3%B6m%20C%2C%20et%20al.%20%0ATransplantation%20of%20human%20embryonic%20stem%20cells%20onto%20a%20%0Apartially%20wounded%20human%20cornea%20in%20vitro.%20Acta%20Ophthalmol%20%0A2013%3B91%3A127-30.
89、Zhu J, Zhang K, Sun Y, et al. Reconstruction of functional
ocular surface by acellular porcine cornea matrix scaffold
and limbal stem cells derived from human embryonic stem
cells. Tissue Eng Part A 2013;19:2412-25.Zhu J, Zhang K, Sun Y, et al. Reconstruction of functional
ocular surface by acellular porcine cornea matrix scaffold
and limbal stem cells derived from human embryonic stem
cells. Tissue Eng Part A 2013;19:2412-25.
90、da Mata Martins TM, da Silva Cunha P, Rodrigues MA, et
al. Epithelial basement membrane of human decellularized
cornea as a suitable substrate for differentiation of
embryonic stem cells into corneal epithelial-like cells.
Mater Sci Eng C Mater Biol Appl 2020;116:111215.da Mata Martins TM, da Silva Cunha P, Rodrigues MA, et
al. Epithelial basement membrane of human decellularized
cornea as a suitable substrate for differentiation of
embryonic stem cells into corneal epithelial-like cells.
Mater Sci Eng C Mater Biol Appl 2020;116:111215.
91、Ahmad S, Stewart R, Yung S, et al. Differentiation of
human embryonic stem cells into corneal epithelial-like
cells by in vitro replication of the corneal epithelial stem
cell niche. Stem Cells 2007;25:1145-55.Ahmad S, Stewart R, Yung S, et al. Differentiation of
human embryonic stem cells into corneal epithelial-like
cells by in vitro replication of the corneal epithelial stem
cell niche. Stem Cells 2007;25:1145-55.
92、Zhang C, Du L, Pang K, et al. Differentiation of
human embryonic stem cells into corneal epithelial
progenitor cells under defined conditions. PLoS One
2017;12:e0183303.Zhang C, Du L, Pang K, et al. Differentiation of
human embryonic stem cells into corneal epithelial
progenitor cells under defined conditions. PLoS One
2017;12:e0183303.
93、Kiskinis E, Eggan K. Progress toward the clinical
application of patient-specific pluripotent stem cells. J Clin
Invest 2010;120:51-9.Kiskinis E, Eggan K. Progress toward the clinical
application of patient-specific pluripotent stem cells. J Clin
Invest 2010;120:51-9.
94、Blazejewska%20EA%2C%20Schl%C3%B6tzer-Schrehardt%20U%2C%20Zenkel%20M%2C%20%0Aet%20al.%20Corneal%20limbal%20microenvironment%20can%20induce%20%0Atransdifferentiation%20of%20hair%20follicle%20stem%20cells%20into%20corneal%20%0Aepithelial-like%20cells.%20Stem%20Cells%202009%3B27%3A642-52.Blazejewska%20EA%2C%20Schl%C3%B6tzer-Schrehardt%20U%2C%20Zenkel%20M%2C%20%0Aet%20al.%20Corneal%20limbal%20microenvironment%20can%20induce%20%0Atransdifferentiation%20of%20hair%20follicle%20stem%20cells%20into%20corneal%20%0Aepithelial-like%20cells.%20Stem%20Cells%202009%3B27%3A642-52.
95、Miki T, Lehmann T, Cai H, et al. Stem cell characteristics
of amniotic epithelial cells. Stem Cells 2005;23:1549-59.Miki T, Lehmann T, Cai H, et al. Stem cell characteristics
of amniotic epithelial cells. Stem Cells 2005;23:1549-59.
96、Miki T. Stem cell characteristics and the therapeutic
potential of amniotic epithelial cells. Am J Reprod
Immunol 2018;80:e13003.Miki T. Stem cell characteristics and the therapeutic
potential of amniotic epithelial cells. Am J Reprod
Immunol 2018;80:e13003.
97、He YG, Alizadeh H, Kinoshita K, et al. Experimental
transplantation of cultured human limbal and amniotic
epithelial cells onto the corneal surface. Cornea
1999;18:570-9.He YG, Alizadeh H, Kinoshita K, et al. Experimental
transplantation of cultured human limbal and amniotic
epithelial cells onto the corneal surface. Cornea
1999;18:570-9.
98、Fatimah SS, Ng SL, Chua KH, et al. Value of human
amniotic epithelial cells in tissue engineering for cornea.
Hum Cell 2010;23:141-51.Fatimah SS, Ng SL, Chua KH, et al. Value of human
amniotic epithelial cells in tissue engineering for cornea.
Hum Cell 2010;23:141-51.
99、Yao M, Chen J, Yang XX, et al. Differentiation of human
amniotic epithelial cells into corneal epithelial-like cells in
vitro. Int J Ophthalmol 2013;6:564-72.Yao M, Chen J, Yang XX, et al. Differentiation of human
amniotic epithelial cells into corneal epithelial-like cells in
vitro. Int J Ophthalmol 2013;6:564-72.
100、Zhou Q, Liu XY, Ruan YX, et al. Construction of corneal
epithelium with human amniotic epithelial cells and repair of limbal deficiency in rabbit models. Hum Cell
2015;28:22-36.Zhou Q, Liu XY, Ruan YX, et al. Construction of corneal
epithelium with human amniotic epithelial cells and repair of limbal deficiency in rabbit models. Hum Cell
2015;28:22-36.
101、Calonge M, Nieto-Miguel T, de la Mata A, et al. Goals
and Challenges of Stem Cell-Based Therapy for Corneal
Blindness Due to Limbal Deficiency. Pharmaceutics
2021;13:1483.Calonge M, Nieto-Miguel T, de la Mata A, et al. Goals
and Challenges of Stem Cell-Based Therapy for Corneal
Blindness Due to Limbal Deficiency. Pharmaceutics
2021;13:1483.