Background and Objective: Subthreshold laser technologies and their applications in ophthalmology have greatly expanded in the past few decades. Initially used for retinal diseases such as central serous chorioretinopathy and diabetic macular edema, subthreshold lasers have recently shown efficacy in the treatment of various types of glaucoma. Our primary objectives are to review the clinical applications of subthreshold laser in the context of glaucoma treatment and discuss the mechanisms of different subthreshold laser techniques, including subthreshold selective laser trabeculoplasty (SSLT), micropulse laser trabeculoplasty (MLT), pattern-scanning laser trabeculoplasty (PSLT), titanium laser trabeculoplasty (TLT), and micropulse transscleral cyclophotocoagulation (MP-TSCPC).
Methods: This was a narrative review compiled from literature of PubMed and Google Scholar. The review was performed from March 2021 to October 2021 and included publications in English. We also included information from web pages to cover details of relevant laser systems. We discuss the history of subthreshold laser, recent advancements in subthreshold techniques, and commercially available systems that provide subthreshold capabilities for glaucoma. We highlight basic science and clinical studies that deepen the understanding of treatment mechanisms and treatment effectiveness in the clinical setting respectively. We review commonly used parameters for each technique and provide comparisons to conventional treatments.
Key Content and Findings: We found five distinct types of subthreshold laser used in the management of glaucoma. Numerous subthreshold laser systems are commercially available and can provide this treatment. Therefore, understanding the differences between subthreshold techniques and laser systems will be critical in utilizing subthreshold laser in the clinical setting.
Conclusions: Traditional laser trabeculoplasty (LT) and cyclophotocoagulation (CPC) have shown effectiveness in the treatment of various types of glaucoma but are associated with visible damage to the underlying tissue and adverse effects. Subthreshold laser systems aim to provide the therapeutic effect found in traditional lasers, while minimizing unwanted treatment related effects. Further clinical studies are needed to evaluate the role of subthreshold lasers in the management of glaucoma.
In 1961, Campbell paved the way as the first physician to use a laser to treat a patient with a detached retina (1). Since then, lasers have been applied to nearly every structure in the eye from the retina to the cornea. Their wide-spread use is largely in part to their minimally invasive nature and high efficacy in treating various disorders. By manipulating the parameters of a given laser, different therapeutic effects may be achieved offering great flexibility and specificity. However, one major challenge that hinders their use is the risk of complications and unwanted tissue damage. The energy of lasers can result in cell death in the targeted region, and the resulting thermal energy can diffuse to nearby tissues causing further damage. Sometimes, this destructive effect is desired. In conventional laser photocoagulation for example, complete damage to the underlying retinal pigment epithelium helps prevent unwanted neovascularization (2). However, in many cases this damage is not desirable, such as in laser treatment in the fovea, where scars may lead to scotomas (3). As a result, much of the research and innovation of lasers in the past few decades have focused on alternative techniques to minimize these unwanted treatment related effects. This need has led to the development of subthreshold lasers. Subthreshold laser has been used primarily for retinal diseases including diabetic macular edema and central serous chorioretinopathy (4-6). The molecular mechanisms behind this technique have been investigated in retinal diseases and have been described as a photostimulation treatment (7-9). In the mid-2000’s, subthreshold lasers began to be applied in treatment for glaucoma with the introduction of micropulse laser trabeculoplasty (MLT) (10). Since then, multiple subthreshold techniques for glaucoma have been introduced. Glaucoma is one of the leading causes of irreversible blindness worldwide, defined as a group of progressive optic neuropathies (11). Various treatment modalities exist for glaucoma including topical medications, surgical intervention, and laser therapy, with the goal to lower intraocular pressure (IOP) in the eye (12). In this article, we review the mechanisms of subthreshold laser techniques in glaucoma, the current systems available for clinical use, and future directions for the use of this technology. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-21-69/rc).
In this narrative review, we compiled and reviewed relevant literature in the English language pertaining to subthreshold lasers systems in glaucoma. We included peer-reviewed articles reporting basic science research and clinical research. We have also included information from commercially available web pages to include relevant information about commercially available subthreshold laser systems. The literature search was done from March 2021 to October 2021. For peer-reviewed articles, we performed our search via PubMed and Google Scholar using combinations of the following search terms: “glaucoma”, “laser”, “subthreshold”, “micropulse”, “transscleral cyclophotocoagulation”, “trabeculoplasty”, “pattern scanning”, “selective laser”, and “titanium” (Table 1).
| Items | Specification |
|---|---|
| Date of search | March 2021–October 2021 |
| Databases and other sources searched | PubMed, Google Scholar, and pertinent publicly available web pages covering commercially available subthreshold laser systems used in glaucoma treatment |
| Search terms used | We included a combination of the following terms for our literature search: “glaucoma”, “laser”, “subthreshold”, “micropulse”, “transscleral cyclophotocoagulation”, “trabeculoplasty”, “pattern scanning”, “selective laser”, and “titanium” |
| Timeframe | Till 2021 |
| Inclusion and exclusion criteria | Relevant literature in the English language were included for this narrative review |
| Selection process | Studies were selected independently by two reviewers and consensus was obtained based on relevant information for the narrative review |
Traditional laser photocoagulation has been a valuable, therapeutic tool to treat various retinal diseases; treatment typically causes a full burn and destruction to the metabolic cells within targeted area (13). This treatment has been widely successful in retina diseases such as diabetic retinopathy (14,15). While the definition of subthreshold laser varies between different sources, one widely accepted interpretation refers to subthreshold laser as a laser technique that provides therapeutic effect while avoiding damage in the treated area (16). The theoretical basis for subthreshold laser is founded under the assumption that scarring and visual changes may not be required to achieve therapeutic effect for certain applications (17). The mechanism of action for subthreshold laser for retinal applications is an area of ongoing investigation. The treatment mechanism has been described as “photostimulation” (7-9). One of the more heavily investigated subthreshold laser techniques has been the subthreshold micropulse laser (SMPL). This technique was developed when the micropulse diode laser was used at low-intensity settings to provide subthreshold effects and was subsequently termed SMPL (4). SMPL achieved subthreshold effects by delivering short pulses of laser instead of a continuous wave (6,18,19). These pulses were in the duration of 100–300 microseconds and had a duty cycle between 5–15% (17). This duty cycle represents the ratio of “on” time to “off” time. This “off” time is what allows the tissue to cool down, thus reducing thermal damage, and distinguishes it from conventional laser photocoagulation. While the exact mechanism of SMPL’s therapeutic effect is still being investigated, a study with mice retinas undergoing SMPL have demonstrated a restoration of oxidant and antioxidant balance (20). De Cillà et al. observed a reduction of thiobarbituric acid reactive substances quantification and expression for superoxide dismutase 1 following SMPL treatment in mice eyes. SMPL therapy in this study also increased Beclin 1 and LC3β, established markers of autophagy in the literature (20,21). Inagaki et al. studied SMPL therapy on a single layer of densely cultured human retinal pigment epithelial cells. SMPL therapy of this cellular culture induced an increased expression of heat shock proteins which persisted for 24 hours following treatment (3). These cellular studies will continue to deepen our understanding of the exact molecular effects of this non-lethal therapy that stimulates expression of certain cellular markers.
SMPL’s effectiveness in the clinical setting parallels these early cellular studies and supports the hypothesis that scarring is not necessary to induce treatment related effects. As a result, other subthreshold techniques have been quickly introduced including selective retina therapy, subthreshold nanosecond laser, endpoint management, and transpupillary thermotherapy (17-19,22). This technology that was originally investigated for retinal diseases has become an area of therapeutic interest in glaucoma. Ingvoldstad et al. reported a study investigating the effects of micropulse diode laser trabeculoplasty (LT) verses traditional argon laser trabeculoplasty (ALT) in open angle glaucoma (OAG) (10). The investigators reported that both methods significantly lowered IOP and that there was no significant difference between the two modalities in the therapeutic effect of lowering IOP. Since this report in the mid 2000s, the study of subthreshold lasers in glaucoma has grown.
Glaucoma is a group of progressive optic neuropathies that can result in visual field loss and is one of the main reasons for irreversible blindness globally. While the etiology of glaucoma is not completely understood, elevated IOP is a significant modifiable risk factor (23). As a result, treatment methods have focused on lowering IOP via medication, surgery, and laser therapy.
In the last four decades, laser therapy has become a standard of care treatment for certain types of glaucoma (24). These laser techniques can be widely generalized into a few major types: laser peripheral iridotomy (LPI), laser peripheral iridoplasty, LT, and cyclophotocoagulation (CPC).
LPI is the most common procedure used for angle closure glaucoma (ACG) and is characterized by using lasers to create a full thickness hole in the iris to allow the posterior chamber to communicate with the anterior chamber; the aim is to nullify pupillary block (25,26). In laser peripheral iridoplasty, also known as gonioplasty, low-energy lasers are applied to the pigmented epithelium of the trabecular meshwork to shrink collagen and to cause a retraction of the peripheral iris root, thus artificially pulling open the angle mechanically (27,28). Iridoplasty is useful in treating eyes with persistent appositional closure post laser iridotomy or in rare instances, in acute angle closure attacks (27,29). LT applies lasers to the drainage angle in the trabecular meshwork (TM) to increase aqueous outflow (24,30-32). By increasing outflow, IOP may be lowered. This technique has primarily been used for OAG. Lastly, CPC utilizes lasers to destroy ciliary body structures to decrease aqueous production and control IOP (30,33). This can be done via a transscleral approach (TSCPC) or an endoscopic approach. Due to the destructive nature of CPC, this technique has conventionally been reserved for cases of refractory glaucoma (24,30).
All these techniques have showed clinical effectiveness in treating glaucoma. However, they are also associated with complications resulting from laser-induced damages to targeted and adjacent tissues (24,34). Therefore, novel research and development has gone into reducing these complications by creating variations of the aforementioned techniques. In this article, we focus on the subthreshold alternatives of these conventional laser therapies. We discuss the underlying mechanisms, their clinical applications in glaucoma, and the current laser systems that provide these subthreshold laser therapies.
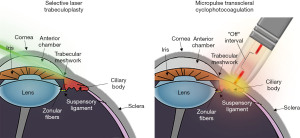
There are five distinct types of subthreshold laser used in the treatment of glaucoma. These include subthreshold selective laser trabeculoplasty (SSLT), MLT, titanium-sapphire laser trabeculoplasty (TLT), pattern-scanning laser trabeculoplasty (PSLT), and micropulse transscleral cyclophotocoagulation (MP-TSCPC) (24). Table 2 provides a summary of these subthreshold laser techniques, clinical applications, and relevant studies (33,35-49). These techniques are all used to reduce IOP, while minimizing damage. SSLT, MLT, TLT, and PSLT are alternatives to the traditional LT and reduce IOP by increasing outflow of aqueous at the TM. MP-TSCPC is a subthreshold variation of conventional TSCPC that reduces IOP by destroying the ciliary body and reducing production of aqueous (Figure 1) (33). Differences in the underlying mechanisms of these treatments differentiate their treatment effect. In this section, we describe each subthreshold laser type, their mechanisms of action, and specific clinical applications.
| Laser | Clinical applications | References |
|---|---|---|
| Subthreshold selective laser trabeculoplasty | Primary open angle glaucoma | (41,46,49) |
| Micropulse laser trabeculoplasty | All types of open angle glaucoma | (39,40,44,45) |
| Pattern scanning laser trabeculoplasty | Primary open angle glaucoma | (47,48) |
| Titanium laser trabeculoplasty | Primary open angle glaucoma | (42,43) |
| Micropulse transscleral cyclophotocoagulation | Primary open angle glaucoma, angle closure glaucoma, refractory open angle glaucoma, secondary open angle glaucoma (adult and pediatric patients) | (33,35-38) |
The standard trabeculoplasty procedure for glaucoma since the 1970s has been ALT (30,50). Lasering of the TM results in mechanical changes and alteration of cellular signaling to enable increased aqueous outflow (51-53). Three distinct theories have been hypothesized to explain the increased outflow (54). One postulated mechanism suggests that shortening and tightening of the TM from coagulative damage results in opening of adjacent intertrabecular spaces. The cellular theory states that laser-induced loss of trabecular cells reduces diseased cells and permits regeneration of new active cells. Lastly, the biochemical theory states that laser radiation changes the biochemical activity of the trabecular cells which leads to alteration of the extracellular matrix. However, due to the cellular damage caused by the high energy and continuous wave modality, ALT is associated with serious complications including IOP spikes and corneal endothelial damage. Minimizing these effects became a primary goal in subsequent innovation of the technique (55).
In 1995, selective laser trabeculoplasty (SLT), which uses a 532 nm Q-switched frequency-doubled Nd:YAG laser that delivers short pulses of 3 ns over a 400 micrometer spot, was introduced (30,40,50,55). Compared to ALT, SLT uses less energy. SLT is performed with 0.1 mJ less than the amount of energy that generates “champagne-like bubbles”, which is usually around 0.6–0.8 mJ. The current protocol of SLT is application of about 100 laser spots over 360-degrees one spot apart. The mechanism of action of SLT includes selective photothermolysis of melanosomes of the melanin-containing cells in the trabecular meshwork. Several mechanisms suggested to be involved in IOP-lowering effect of SLT, including mechanical debridement, activation of specific molecular pathways, and inflammation, none of them solely explains the action of this procedure (56-58). Due to its increased benefits and safety profile over ALT, SLT has replaced ALT after gaining Food and Drug Administration (FDA) approval in 2001 (50). The increased safety profile is a result of the pulsing and lower energy used in SLT, which is in contrast with the continuous wave laser used in ALT. The pulsing allows for selective targeting of TM cells without producing collateral thermal damage to non-pigmented cells. The intervals of “off” time provides enough time for the cell to cool and prevent heat generation (55). SLT has been shown to produce IOP lowering effects that are comparable with ALT with lower post-operative inflammation (24,30). The reduction of IOP of 20% or more has been considered to be a criterion for the efficacy of this procedure. As in the case with ALT, there is continued diminishing effects with subsequent treatment, lowering the long-term effectiveness of treatment (59). However, the absence of tissue damage and better safety profile allows SLT to be repeated to maintain target IOP (32).
In the “LiGHT” multicenter randomized controlled trial, SLT was compared to eye drops for first-line treatment of OAG in 718 patients (60). Over 36 months, eyes of patients who received SLT were more likely to stay within their target IOP, and SLT showed a greater cost-effectivity than eye drops with a 97% probability. As a result of this study, SLT should be considered a first-line treatment option for OAG.
While, SLT decreases the tissue damage caused by trabeculoplasty, its categorization as a subthreshold laser is debatable as it causes selective cell death in the targeted tissue (61). It is worth noting, that SLT remains a safe procedure with limited damage to trabecular meshwork observed after high energy procedures (up to 2 mJ per shot) (62). Nonetheless, the widespread adoption of SLT paved the way for future subthreshold techniques that minimize the unwanted treatment related effects.
As the concept of subthreshold laser for trabeculoplasty expanded, new applications in glaucoma procedures also arose including SSLT, MLT, TLT, and PSLT (24,34,49,63). These techniques utilize different methods to lower IOP and minimize damage to the adjacent tissues.
SSLT, also referred to as low-energy SLT was first introduced in 2011 (46). The technique is a natural extension to traditional SLT where the goal is to further minimize damage while retaining the IOP-lowering effects (49,64). While SLT uses lower energy than ALT, SSLT uses even lower energy than that of SLT. SSLT is performed with 0.3–0.4 mJ or two-thirds of the energy used in SLT (41,49). This technology mimics that of SLT, using a Nd:YAG laser system with 532 nm wavelengths, 3 ns pulse width, and 400 micrometer spot size. SSLT only requires modification of power, so most laser systems that can perform SLT can also perform this technique, since the minimum energy allowed by manufacturers in SLT devices is around 0.3 mJ.
The mechanism of SLT is poorly understood, and the mechanism of SSLT is even less so. A likely hypothesis is one that parallels the biochemical theory of SLT, increased trabecular outflow resulting from modified cellular activity and cytokine release (65). Additionally, monocyte recruitment to the TM following SLT may increase tissue permeability as debris obstructing TM outflow is phagocytized (66). Regardless of the IOP-lowering mechanism, the reduction in overall energy compared to SLT results in a lack of “champagne-like bubble” formation (49). This lack in cellular damage characterizes the subthreshold quality of SSLT.
SSLT and low-energy SLT has been explored in the treatment of primary open angle glaucoma (POAG). A recent prospective observational case series found that while SSLT used significantly lower initial and total energy dosage, there was no difference in the amount of inflammation, reduction in IOP, or success rate in controlling IOP between SSLT and SLT (49). Other studies have also concluded that SLT with reduced energy lowers IOP with fewer complications (41,46). One retrospective study found that low-energy SLT treatment was particularly effective in younger POAG patients (64). Despite the promising preliminary studies, more research must be performed to assess the clinical viability of SSLT. In response to this need, the COAST Trial, a randomized clinical trial with 640 participants, will be conducted to compare the effects of low-energy SLT, defined as 0.3–0.4 mJ per spot, and standard energy SLT, 0.8 mJ up to champagne bubble visualization per spot (67). This trial will evaluate these treatments for the management of mild and moderate POAG.
MLT is the trabeculoplasty variant of SMPL and a subthreshold alternative to ALT. As SMPL showed effectiveness in retinal pathology treatment in the 2000s, the same strategy was applied to trabeculoplasty. Similar to SMPL, MLT achieves subthreshold effects by breaking a continuous laser into short pulses with enough time in between the pulses to allow for cooling of the targeted pigmented cells (10). Due to the recency of the technology, parameters for optimal treatment are ill-defined (34). However, studies have been done with the following settings: 532, 577, and 810 nm wavelength lasers; spot sizes of 75, 125, 200, and 300 micrometers; duty cycle of 15%; spot duration of 200 and 300 microseconds; and a treatment area of TM at 180 and 360 degrees (Table 3) (39,40,44,45,68-76). There is no standardized protocol for MLT settings, but recent studies recommend 532 and 577 nm lasers with a spot size of 300 micrometers and a 360-degree treatment area (34,45,76-78).
| Reference | Study year | Wavelength (nm) | Power (mW) | Spot size(s) (μm) | Spot duration (μs) | Duty cycle | Trabecular meshwork area | Treatment group |
|---|---|---|---|---|---|---|---|---|
| Kakihara et al. (76) | 2021 | 577 | 700–1,000 | 300 | 300 | 15% | 360° | OAG |
| Sun et al. (45) | 2021 | 532 | 1,000 | 300 | 300 | 15% | 360° | OAG |
| Hirabayashi et al. (68) | 2019 | 532 | 1,000 | 300 | 300 | 15% | 360° | OAG |
| Hong et al. (69) | 2019 | 532 | 1,000 | 300 | 300 | 15% | 360° | POAG |
| Valera-Cornejo et al. (70) | 2018 | 532 | 1,000 | 300 | 300 | 15% | 360° | OAG |
| Abramowitz et al. (39) | 2018 | 577 | 1,000 | 300 | 300 | 15% | 360° | OAG |
| De León et al. (44) | 2017 | 577 | 1,000 | 300 | 300 | 15% | 360° | OAG/OHT |
| Abouhussein et al. (75) | 2016 | 577 | 1,000 | 300 | 300 | 15% | 360° | POAG |
| Babalola et al. (71) | 2015 | 810 | 1,000 | 75, 125, 200 | 200 | 15% | Inferior 180° | POAG |
| Lee et al. (72) | 2015 | 577 | 1,000 | 300 | 300 | 15% | 360° | OAG |
| Rantala et al. (73) | 2012 | 810 | 2,000 | 300 | 200 | 15% | Inferior 180° | OAG |
| Fea et al. (40) | 2008 | 810 | 2,000 | 200 | 200 | 15% | Inferior 180° | OAG |
| Detry-Morel et al. (74) | 2008 | 810 | 2,000 | 300 | 200 | 15% | Inferior 180° | OAG |
OAG, open angle glaucoma; POAG, primary open angle glaucoma; OHT, ocular hypertension.
The underlying mechanism of MLT is unknown, but it is hypothesized that MLT utilizes a cellular biochemical reaction like ALT and SLT (24,34,53). However, the lower energy levels of MLT over ALT and SLT make it a desirable option to minimize side effects. While SLT selectively damages pigmented TM cells leading to postoperative inflammation and IOP spikes, MLT thermally affects the trabecular cells without destruction (45,79). The underlying goal of MLT is to stimulate the biological response via cellular changes and cytokine release (53,80). MLT does not lead to any morphologic changes to the TM on scanning electron microscopy, and there are no visible signs of treatment during the procedure (39). Since there are no visible treatment endpoints for MLT, titration is difficult and there is a steep learning curve associated with using this technology (30,75,76). If an automated solution with in-built tissue monitoring could be used, like that seen in novel selective retina therapy treatments, these barriers to use may be overcome (81).
Clinically, MLT has been tested for the treatment of all types of OAG. In a prospective interventional case series, eyes with uncontrolled OAG were treated with MLT and IOP was measured and analyzed at 1 hour, 1 day, 1 week, 3, 6, 9, and 12 months post-treatment (40). The IOP was significantly lowered throughout follow-up and there was no increase in flare or formation of peripheral anterior synechiae. One IOP spike was observed. Following this initial study, further studies were conducted to compare MLT with ALT and SLT. In a recent retrospective cohort study, patients receiving SLT and MLT were compared (45). A similar success rate was reported for both groups, but the SLT group had on average more transient IOP spikes at 1-hour post-laser. In a randomized prospective study, patients receiving SLT and MLT had similar reduction in IOP at 36–52 weeks follow-up (39). However, the MLT group reported less pain during and after the procedure. Additionally, a controlled, prospective, longitudinal, clinical trial was performed to compare SLT to MLT, which showed similar reductions in IOP for the two groups (44). A recent retrospective study looked at the effectiveness of MLT when performed by glaucoma specialists of varying expertise to determine the difficulty of applying the new treatment (76). The researchers noted a statistically significant difference in the mean survival rates of IOP reduction when the procedures were performed by an experienced specialist versus an unexperienced specialist (76). In general, recent studies indicate similar effectiveness between MLT and SLT for the treatment of all types of OAG, but with reduced complications and pain in MLT (34). The treatment group for these studies are summarized in Table 3.
PSLT is a solution to traditional trabeculoplasty that takes a different approach to reach subthreshold activity than SSLT or MLT, using an algorithm to apply a sequence of laser pulses to the TM for the treatment of POAG (31,82). Originally developed as a part of Topcon’s PASCAL system, PSLT simplifies treatment by allowing computer-guidance to calculate the alignment between laser spots, minimizing overlap and excessive gaps introduced by human error (82). This computer driven algorithm is one of the key advantages of the PSLT system, as it can theoretically provide more precise treatment. The patterns readily align to the TM and allow for quick application, which reduces treatment time and increases patient comfort (83). PSLT differs from SLT by using a higher pulse energy, around 3.4 mJ, over a 100-micrometer spot size. During treatment 3 rows of 13 spots each are placed with proper spacing using a computer guided algorithm. This process is repeated 32 times for a 360-degree trabeculoplasty (82). To achieve subthreshold effects, the laser must be titrated with 10 ms pulse lasers. Following titration, the pulse is shortened to 5 ms for treatment. In order to maximize absorption in the TM, a wavelength of 532 or 577 nm is used (31).
Like MLT, the underlying mechanism of PSLT is unknown but is hypothesized to be like ALT and SLT. Furthermore, the pulse energy, spot size, and pulse duration are closer to what is used in MLT. As a result, the underlying mechanism is presumed to be similar, inducing cellular change and cytokine release. However, there is no definitive evidence on the mechanism of action. In SLT, the pulse duration of 5 ns restricts the energy to the pigmented cells, causing selective cell death (84). However, in PSLT, the longer 5 ms pulse duration allows the heat to diffuse approximately 50 micrometers (47). This reduces cell death in the targeted pigmented cells but yields greater thermal damage in surrounding cells. PSLT does not cause immediate visual changes, which would typically make the application of treatment difficult for a clinician as was observed in MLT. However, in PSLT, the computer-guided algorithm prevents this and reduces errors associated with the adoption of new technologies (82). A study comparing PSLT and ALT in cats’ TM revealed that subthreshold PSLT resulted in the thinning of uveal meshwork and denudation of endothelial cells (85). The study concluded that at subthreshold power, PSLT caused less damage to the TM than ALT but did not prevent late scarring in the TM (85). This calls into question whether PSLT is truly subthreshold due to the presence of cell damage.
In an initial preliminary clinical case series published in 2010, 25 patients with POAG received PLST (47). A significant reduction in IOP was reported during 6 months of follow-up. Following this, ALT and PSLT were compared in a retrospective study published in 2014 (86). There was no difference in IOP reduction between patients treated with PSLT and ALT at a mean follow-up of 8.2 weeks. These studies prompted evaluation of PSLT efficacy and safety in larger randomized trials. In one randomized clinical trial, patients with POAG or ocular hypertension were either treated with PSLT or SLT (48). During successive follow-ups, there were no differences in IOP reduction, visual field mean deviation, average retinal nerve fiber layer thickness, corneal endothelial cell count, or visual acuity between the two groups. This similarity in safety and efficacy is also supported by other randomized control trials and retrospective studies (83,84,87,88). Interestingly, researchers found that PSLT was better tolerated by patients (83). Despite the scarring observed in cat eyes, there were no pressure spikes or inflammation following PSLT in clinical studies (47). These studies show promise that PSLT treatment for POAG may be a safe and efficacious alternative to traditional SLT.
TLT is yet another variant of traditional LT that utilizes a unique titanium: sapphire laser to achieve treatment results (42). A 790 or 800 nm laser can be used to deliver energy pulses ranging from 5 to 10 microseconds over a 200 nm spot size (43). Like SLT, TLT is performed via 95 to 105 spots in a 360-degree range about the TM. Although the energy is greater than that used SLT, 50 vs. 0.9 mJ, the energy is delivered over a longer period resulting in less disruption and increased thermal effect (31). Currently the only system to have FDA clearance to perform TLT is the SOLX 790 from SOLX (89). Therefore, information on TLT is relatively low compared to other subthreshold laser methods for glaucoma.
The mechanism is assumed to have overlap with SLT, albeit with several additional properties. The increased wavelength (790–800 nm) of TLT is hypothesized to provide deeper penetration and better target the juxtacanalicular meshwork as well as the inner wall of the canal of Schlemm (43). Pigmented phagocytic cells absorb the laser, which allows for sparing of the TM (31). The current understanding of TLT’s mechanism of action is thought to induce change in these phagocytic cells and cytokine release (31,50). SLT laser will typically penetrate 20–50 micrometers into the tissue, while TLT lasers can penetrate 200 micrometers. This allows the TLT laser to penetrate and reach more of the TM tissue. Additionally, the TLT laser uses a much longer pulse duration, minimizing damage to targeted cells (31). A scanning electron microscopy study showed that TLT did not cause structural damage to the TM (90). Additionally, it is hypothesized that the laser is selectively absorbed by pigmented phagocytic cells. This property allows for outflow changes without damaging TM cells, increasing the chances of successful repeat treatment (43).
TLT has been studied in the treatment of POAG. In 2009, a pilot-study with 37 subjects was published comparing TLT and ALT with respect to treatment of POAG (42). Both treatments led to a reduction in IOP, and the difference was not statistically significant. In 2016, a two-year randomized prospective clinical trial with 37 subjects was published comparing TLT and SLT in the treatment of POAG (43). IOP decreased by 35% in the TLT group and 25% in the SLT group. The difference was not statistically significant. In both studies, no long-term complications occurred in patients who received TLT or SLT. Initial data shows promise that TLT can achieve similar efficacy to SLT and ALT in the treatment of POAG. However, larger scale randomized studies must be conducted before definitive comparisons can be drawn to standard of care treatments and TLT’s hypothesized improved safety profile.
MP-TSCPC uses a different technique and mechanism than the subthreshold lasers previously described. Instead of increasing outflow of aqueous, MP-TSCPC uses transscleral cyclophotocoagulation (TSCPC) to decrease aqueous production. In 1972, TSCPC was developed and used to treat refractory glaucoma by destroying parts of the aqueous producing ciliary body (91). Due to the inherent damaging effects of the procedure, it is usually reserved for patients with poor visual potential (92,93). Recent studies have explored TSCPC use in patients with good visual acuity (94). However, there is insufficient evidence to conclude its effectiveness and safety in patients with non-refractory glaucoma (95). Traditionally, TSCPC is performed with a continuous wave laser (CW-TSCPC), a power between 1,500–2,000 mW, and a duration of 2 seconds. Although initial lasers used 693 and 1,064 nm wavelengths, current lasers use 810 nm semiconductor lasers. This is due to the increased portability and decreased complication rate of 810 nm lasers (96). Contrary to LT, which targets the TM to increase aqueous outflow, TSCPC targets the ciliary body to reduce aqueous production. Unfortunately, this treatment has been associated with serious complications such as hypotony, phthisis bulbi, visual loss, and sympathetic ophthalmia (92,93). Additionally, TSCPC is a painful procedure which may require an operation room to be performed as well as regional anesthesia.
To minimize such risks, MP-TSCPC was introduced in 2010 (38). This novel technique extends the use of subthreshold lasers to TSCPC by using repetitive, short pulses of laser with “on” and “off” times to allow for thermal cooling in between pulses (34,38,63). Laser settings have varied between studies, but typically a 31.3% duty cycle with an on time of 0.5 ms is used while applying spots from 9:30 to 2:30 for the superior quadrant and 3:30 to 8:30 for the inferior quadrant (34,38,63). The 3 and 9 o’clock positions are avoided to reduce the risk of ciliary neurovascular injury (97). An 810 nm wavelength is used in order to maximize absorption by the melanin of the pigmented ciliary body (34). Total energy applied also varied between studies, ranging from 62 to 225 J (33). Since MP-TSCPC has a reduced risk profile, while maintaining similar efficacy to CW-TSCPC, it can be used earlier in the treatment regime and is no longer reserved for refractory and end-stage glaucoma (33). Moreover, MP-TSCPC is a well-tolerated procedure for the patient and was proposed as an in-office procedure.
While the underlying mechanisms is not fully understood, several mechanisms have been suggested (33). The leading hypothesis is that the laser destroys parts of the ciliary body to minimize aqueous humor production and activate certain biochemical cascades (98,99). This occurs during the “on” time of the laser, where laser energy causes coagulation at the pigmented ciliary epithelium. At the microscopic level, MP-TSCPC shows coagulation of collagen and destruction of the ciliary stroma, albeit far less frequently than in CW-TSCPC. Additionally, full-thickness destruction in the ciliary body is not observed in MP-TSCPC (100). The lack of significant damage to the ciliary body suggest that additional processes lead to the decrease in IOP seen in MP-TSCPC (33). One suggested mechanism is increased uveoscleral outflow as a result of prostaglandin release (100). This has been observed in traditional TSCPC but has not been shown in MP-TSCPC (98,101). Although MP-TSCPC minimizes damage to the targeted tissue by utilizing “off” time, cellular damage is still present, so the technique may not truly be subthreshold.
Clinically, MP-TSCPC has been tested rather extensively in recent years. It has been indicated for a wide variety of glaucoma stages including POAG, ACG, refractory OAG, and various secondary OAGs for both the adults and pediatric populations (33). Initial studies focused on the applications of MP-TSCPC in uncontrolled and refractory glaucoma as was indicated for the use of CW-TSCPC (33). In an early prospective interventional case series, refractory glaucoma was treated with MP-TSCPC (38). Follow-up at 18 months after treatment showed a significant decrease in mean IOP and no hypotony or loss of best-corrected visual acuity. In a prospective, non-randomized interventional case series, eyes with various types of glaucoma, the most common being neovascular glaucoma, were treated with MP-TSCPC (36). At 12-month follow-up, there was a significant reduction in IOP with no significant adverse events or complications. In an interventional case series, eyes with POAG were treated with MP-TSCPC (102). IOP was significantly decreased at 3-, 6-, and 12-month follow-ups, although roughly a third of the eyes required further surgical intervention. Increased IOP reduction was related to higher power settings. These studies as well as others in the literature have indicated the IOP lowering effects of MP-TSCPC for various types of glaucoma (103-107).
Other studies have directly compared the use of MP-TSCPC and CW-TSCPC. In a randomized comparative study, 48 patients with refractory glaucoma were randomized to either receive MP-TSCPC or CW-TSCPC (37). At 18 months, a successful outcome was reported in 52% and 30% of patients who received MP-TSCPC and CW-TSCPC respectively. This difference was not statistically significant. In a prospective study, 45 eyes from pediatric patients with refractory glaucoma either received MP-TSCPC or CW-TSCPC (35). While both treatments were effective in lowering IOP, the rate of complications were fewer in patients receiving MP-TSCPC. Other studies also confirm the increased safety profile of MP-TSCPC and even suggest the possibility of using MP-TSCPC before patients reach the stage of refractory glaucoma (63). However, in one case study, a patient presented with intraocular lens subluxation 5-weeks following MP-TSCPC (108). Overall, MP-TSCPC shows great promise in changing the modern approach to laser treatment for glaucoma, but further randomized comparative studies with other standard of care treatments should be performed.
Subthreshold laser systems for glaucoma applications have greatly changed in the past two decades as new technologies emerge and companies compete to design better products with reduced complications. Furthermore, the parameters and techniques used are constantly being tweaked as more data becomes available on treatment outcomes and new research deepens understanding of underlying mechanisms. Innovation has also taken place in the form of add-ons and assistive technologies that improve the user experience. Many devices on the market currently advertise multi-functionality, offering solutions for glaucoma, cataracts, and retinal pathologies all from a single device. In this section, we aim to provide an overview of the laser systems offered by different companies that have subthreshold laser capabilities for glaucoma treatment. This overview is meant to provide clinicians with a summary of commercially available systems. While the systems discussed will often have additional capabilities, the focus will be on their subthreshold solutions for glaucoma. Table 4 compares the functionality and specification of several subthreshold laser systems (77,82,89,109-115).
| Parameter | Device | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclo G6 | IQ 532 | IQ 577 | IQ 810 | OcuLight SLx | PASCAL Synthesis 532 | PASCAL Synthesis TwinStar | Navilas Prime | Navilas Pro | Vitra 810 | DeepLight 790 Laser | |
| Weight | 4.8 kg | 9.0 kg | 9.0 kg | 5.0 kg | 6.3 kg | 15.0 kg | 15.0 kg | – | – | 5.6 kg | 81 kg |
| Laser type | Solid-state | Solid-state | Solid-state | Solid-state | Solid-state | OPS | OPS | OPS | OPS | Solid-state | TS |
| Digital display | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | – |
| Touch screen | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | – |
| Pattern | N | N | N | N | N | Y | Y | Y | Y | Y | – |
| Recording | N | N | N | N | N | N | N | Y | N | N | – |
| Technology | MP-TSCPC | MLT | MLT | MLT | MLT | PSLT | PSLT | MLT, PSLT | MLT, PSLT | MP-TSCPC | TLT |
| Pulse length | 0.05–1.0 ms | 0.05–1 ms | 0.05–1.0 ms | 0.025–1 ms | 0.1–1 ms | – | – | – | – | ≥10 ms | 0.007 ms |
| Pulse interval | 1.0–10.0 ms | 1.0–10.0 ms | 1.0–10.0 ms | 1–9.5 ms | 1.0–10.0 ms | – | – | – | – | – | – |
| Duty cycle | 0.5–50% | 5%, 10%, 15% | 5%, 10%, 15% | 5%, 10%, 15% | 0.4–50% | – | – | – | – | 5% to 35% | – |
| Power/energy | 50–3,000 mW | 400–1,200 mW | 400–1,200 mW | 0–2,000 mW | 0–3,000 mW | 3.4 mJ | 3.4 mJ | 50–2,000 mW | 50–2,000 mW | 0–3,000 mW | 30–120 mJ |
| Wavelength | 810 nm | 532 nm | 577 nm | 810 nm | 810 nm | 532 nm | 577 nm | 577 nm | 577 nm | 810 nm | 790 nm |
OPS, optically pumped semiconductor; TS, titanium: sapphire; MP-TSCPC, micropulse transscleral cyclophotocoagulation; MLT, micropulse laser trabeculoplasty; PSLT, pattern-scanning laser trabeculoplasty; TLT, titanium laser trabeculoplasty.
IRIDEX Corporation (Mountain View, CA, USA) is a medical device company focused on developing, manufacturing, and marketing laser-based systems for ophthalmic applications (116). The company’s OcuLight SLx System was one of the first subthreshold laser systems used in the clinical setting and helped pave the way for subthreshold laser use in ophthalmology. In a nonrandomized clinical study, the laser was effective in the treatment of patients with choroidal neovascularization, macular edema from branch retinal vein occlusion, and diabetic macular edema (117). This led to a breakthrough in new companies and systems innovating and developing subthreshold lasers.
In the 2000s, the company branched from retinal treatments to also introduce MicroPulse technology for glaucoma applications (113). This led to the development of MLT and MP-TSCPC. For the integration of MLT, the company provided a special MLT lens that allowed retinal lasers to be applied to the TM for trabeculoplasty. This expanded the treatment options provided by their existing devices, shown in Table 4. The MLT device has the advantage of flexibility as clinicians can use existing systems for glaucoma treatment. However, this comes with the drawback that the system is not tailored for this use case from the ground-up. For the integration of MP-TSCPC, the company went with a different approach by combining their Cyclo G6 system with a MicroPulse P3 delivery device to provide treatment (113). This system is designed specifically to provide MP-TSCPC and has extensive publicly available information to help clinicians get started. Recently, IRIDEX also acquired Topcon’s PASCAL laser technology (118). The PASCAL line of systems provides retinal and glaucoma treatments. They utilize a unique EndPoint management algorithm to titrate energy for retinal applications, and a pattern-scanning algorithm to provide PSLT for glaucoma (82).
OD-OS (Teltow, Germany) is an ophthalmic laser subsidiary of SensoMotoric Instruments GmbH that specializes in retinal laser applications through their Retina Navigation platform (119). This platform, which is integrated in their Navilas Laser System products, combines imaging, planning, treatment, and documentation into a streamlined format, which is a distinguishing feature of this laser system (19). This is made possible by allowing systemic integration of real-time imaging, digital planning, treatment overlay, and documentation. Additionally, caution zones are displayed during treatment to minimize risk and improve safety. The company currently offers products that can provide SMPL, MLT, and PSLT, albeit the last of which has not be evaluated in a published study (111,112,120).
Quantel Medical (Cournon d’Auvergne, France), a subsidiary Lumibird, is an ophthalmic device company that focuses on developing lasers for both retinal and anterior chamber applications (121). In 2009, Quantel Medical released the Supra Scan 577, which was the first 577 nm multispot pattern scanning laser with subthreshold capabilities. One of the primary advantages of the devices offered by the company is the multi-functionality of their laser systems. For example, the Optimis Fusion device allows for YAG capsulotomy, peripheral iridotomy, trabeculoplasty, SSLT, and retinal photocoagulation to be performed from one device (122). This device can also be adjusted to perform SSLT. Quantel Medical also offers MP-TSCPC solutions via the company’s SubCyclo technology in devices such as the Vitra 810 system (105,115). The device is portable and compatible with many of the company’s other products, but it is not available universally.
SOLX Inc. (Waltham, MA, USA) is an ophthalmic device company that focuses on glaucoma devices. The company introduced the DeepLight Titanium Sapphire Laser Technology, which led to the development of the SOLX 790 Laser system (123,124). This DeepLight system provides TLT therapy. Additionally, SOLX pioneered the SOLX Gold Shunt, a gold tube shunt to lower IOP in glaucoma patients (125). In 2006, these products were renamed to DeepLight 790 Titanium Sapphire Laser and DeepLight Gold Micro-Shunt (125). The literature surrounding these systems is limited.
Preliminary data suggests that emerging subthreshold laser therapies for glaucoma, such as SSLT, MLT, PSLT, TLT, and MP-TSCPC may be effective and safe in treating various types of glaucoma. The goal of these techniques is to retain the efficacy of treatment while minimizing laser-induced adverse effects and complications, such as: IOP spikes, corneal endothelial damage, hypotony, phthisis bulbi, pain, etc. Each technique offers a different approach to achieve this goal, which may prove beneficial when treating various types of glaucoma. SSLT and TLT have both shown promise but require larger randomized control trials to support further development. PSLT and MLT have both seen a rise in research since 2018 (6 and 22 articles respectively on PubMed) with prospective trials showing similar efficacy between these subthreshold techniques and SLT. MP-TSCPC has also seen a rapid growth in clinical studies and research since 2018 (79 articles on PubMed). Trials show IOP lowering effects of MP-TSCPC treatment for various types of glaucoma, particularly refractory glaucoma, and similar effectiveness to CW-TSCPC with fewer complications.
Many of these techniques can be performed using existing SLT devices or commercial products from ophthalmic laser manufacturing companies, making the transition for clinicians easier. However, additional comparative studies must be performed to support subthreshold lasers as an effective alternative for glaucoma treatment. Furthermore, larger studies and long-term monitoring must be conducted to standardize technique parameters and methods.