Charles Bonnet syndrome (CBS), named after the Genevan natural historian Charles Bonnet, is the occurrence of complex visual hallucinations in patients with visual impairment and partial or complete insight into the unreal nature of the visual experience (1). As a consequence of his own gradual vision loss, Bonnet’s interest turned to philosophy and mental health and in year 1769, he published a book on his observations (Essai Analytique sur les Facultés de l’Ame; English: Analytical Essays Concerning the Faculties of the Mind). In this book, he described his mentally healthy, but blind 87-year-old grandfather, who saw faces, birds, horse carriages, and buildings, and was perfectly aware that these observations were unreal (2). The term CBS was coined by the French neurologist Georges de Morsier in a publication in 1967 titled, ‘Visual hallucinations in the aged without mental deficiency’ (3). The pathophysiology of CBS is not completely understood, although it is thought to be an interplay of higher cortical level inputs producing complex visual hallucinations (4).
Although many ophthalmologists have background knowledge of CBS, one common presumption is that it is rarely seen in eye clinics. However, studies suggest that CBS may be more common than anticipated when patients are systematically interviewed for presence of CBS (5,6). CBS has also been described in numerous other less common ophthalmic conditions (7-11). One explanation for the discrepancy in the perceived rarity of this condition and its actually relatively common occurrence may be that the patients might not discuss visual hallucinations with their ophthalmologist for various reasons, e.g., taboo subject in many cultures, self-perceived irrelevance to their eye condition, association of visual hallucinations with neurodegenerative or psychiatric disease and unawareness of CBS (4,12).
The topic of CBS is receiving increasingly more attention (13). The latest revision of the International Classification of Diseases version 11 (ICD-11) by the World Health Organization has allocated a specific diagnose code for CBS: 9D56 Visual release hallucinations “Charles Bonnet syndrome, also called visual release hallucinations, refers to the experience of complex visual hallucinations in a person who has experienced partial or complete loss of vision. Hallucinations are exclusively visual, usually temporary, and unrelated to mental and behavioural disorders.” (14). Furthermore, patient support groups specific to CBS have emerged and work with healthcare stakeholders for improving awareness, diagnosis, support, and research funding specific to CBS (13). However, one question remains unanswered, and is of high importance for stakeholders when planning and allocating resources to CBS: Exactly how common is CBS in patients with low vision? In this systematic review and meta-analysis, we aim to answer this question through a systematic review of published studies with a meta-analysis to provide a summary prevalence estimate. We present the following article in accordance with the PRISMA checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-21-37/rc) (15).
Our protocol was registered in the PROSPERO database (reg. no. CRD42021255021) (https://cdn.amegroups.cn/static/public/aes-21-37-1.pdf) (16). According to Danish Law, institutional review board approval is not relevant for systematic reviews.
Eligible studies were defined as those on a population of low vision patients wherein the prevalence of CBS was evaluated (without any further restriction on the method for diagnosis of CBS). We focused on studies considering a consecutive group of low vision patients, i.e., not studies of specific eye diseases but rather prevalence studies in low vision clinics or population-based studies of low vision individuals. No restriction was enforced on study design, but we expected observational cross-sectional studies due to the nature of our research question. Studies only reported as conference abstract were considered eligible. We did not consider case reports or review papers without original data. We did not restrict studies based on geography or journal, but only considered studies disseminated in English language.
We searched the following literature databases: PubMed/MEDLINE, EMBASE, Web of Science Core Collection, BIOSIS Previews, Current Contents Connect, Data Citation Index, Derwent Innovations Index, KCI-Korean Journal Database, Russian Science Citation Index, SciELO Citation Index, and the Cochrane Central. Details of our search strategy in individual databases are available as https://cdn.amegroups.cn/static/public/aes-21-37-2.pdf. The search was performed on April 21st, 2021.
One author (Y Subhi) examined the title and abstracts of all identified records and removed those deemed as obviously irrelevant or duplicates. Two authors (Y Subhi and MA Nielsen) then independently examined remaining references in full text for eligibility. References from these studies were also screened for any additional relevant studies. Disagreements in study selection were discussed between the authors, and where consensus could not be reached a third author (A Singh) would be included for final decision making.
We extracted data on study characteristics, population characteristics, diagnosis of CBS and prevalence of CBS using pre-designed study data extraction form. If studies reported data on several groups, we focused our data extraction for the quantitative analyses on those with best-corrected visual impairment of the best-seeing eye of 6/18 or worse and on adults aged ≥40 years. We enforced age restriction on data to focus the data analysis on the acquired causes of visual impairment, as the relationship between visual impairment from childhood and visual hallucinations is hypothesized to be more complex and different from those acquired in later age. If these visual acuity or age criteria could not be fulfilled, we extracted data where we anticipated that the majority of cases would fulfill these criteria. Since we anticipated that the majority of studies would be of cross-sectional design, we evaluated quality of eligible studies using relevant items from the Agency for Healthcare Research and Quality (AHRQ) checklist for Cross-Sectional Studies, which is the recommended tool for evaluating cross-sectional studies (17).
Two authors (Y Subhi and DAR Scott) extracted data and evaluated risk of bias of individual studies in an independent fashion. Any disagreements between the authors were discussed and where consensus could not be reached a third author (A Singh) would be included for final decision making.
Eligible studies were described in text and summarized in tables for a qualitative synthesis. The primary outcome measure was the prevalence of CBS in low vision patients. Meta-analysis was made using MetaXL 5.3 (EpiGear International, Sunrise Beach, QLD, Australia) for Microsoft Excel 2013 (Microsoft, Redmont, WA, USA). We used the random-effects model to account for potential heterogeneity across studies. In prevalence meta-analyses, caution must be shown when numbers are close to the extremes (0% or 100%) because of variance instability, which results in studies getting erroneous weights (18). Therefore, all prevalence numbers were transformed using the double arcsine method for analysis and then back-transformed for interpretation (18). Heterogeneity was assessed using the Cochran’s Q and I2 (19). We used a Funnel plot to identify skewed results and evaluate possible publication bias (20). Individual prevalence estimates and the pooled summary measure prevalence estimate was presented using a Forest plot (21). Sensitivity analysis was made by removing studies in turn and evaluating the magnitude of the change of the results.
The literature search across multiple databases identified a total of 350 records, and three additional records was known to us a priori. After discarding duplicates (n=71), obviously irrelevant records (n=266), 16 records remained and were screened in full text. Of these, five were excluded because they did not fulfill our eligibility criteria (Figure 1). Eleven records were deemed eligible and included for the qualitative and quantitative review.
The eleven studies included in the review collectively summarized data on 4,521 individuals with visual impairment. Study characteristics and definitions used in the individual studies are summarized in Table 1. Studies were designed as cross-sectional (n=8) or cohort studies (n=3); however, all reported prevalence data were cross-sectional data. Studies were based on populations in North America (n=4), Europe (n=4), Asia (n=2), and Oceania (n=1). Apart from the study from India by Marmamula et al. (2019) where the majority were diagnosed with cataract (22), and two studies with no data on diagnoses, the majority of patients in the remaining studies had a clinical diagnosis of age-related macular degeneration (AMD). Diagnosis of CBS was based on screening questions or interviews, which in positive cases often were explored in more detail with follow-up questions or further interviews (Table 1). Most studies either had normal mental state as a participant eligibility criterion or as a part of the diagnosis of CBS (Table 1). The diagnostic definition of CBS was not outlined in six studies, and based on a set of criteria for the diagnosis of CBS in two studies, based on whether or not visual hallucinations were complex in two studies, based on psychiatrist evaluation without any further details in one study (Table 1).
| Reference | Country | Study design | Population description | Sample size | Demographics | Clinical diagnoses | Diagnosis of CBS | Definition of CBS |
|---|---|---|---|---|---|---|---|---|
| Crumbliss et al. 2008 | USA | Cohort | New attendees of a low vision rehabilitation clinic; mean BCVA in best-seeing eye was 20/175 and 20/114 in those with and without CBS, respectively; all participants underwent MMSE, but this was not used for study eligibility | 50 | Mean age 74 years (range, 23 to 95 years); 48% were female | No data for the entire study population, but patients with follow-up data had mostly AMD (64%), and the rest optic nerve pathology, BRVO or macular edema | Participants were administered an 11-item questionnaire designed to determine whether they had experienced visual hallucinations typical of CBS | Unclear |
| Elflein et al. 2016 | Germany | Cross-sectional | Attendees recruited from an institution for rehabilitation of the blind and the visually impaired; of the 324 participants in total, we focused on visually impaired aged >40 years; all participants had to pass a modified MMSE for the blind and DemTest | 81 | Median age 75 years (range, 41 to 99 years); 62% were female | No data | A standardized, detailed interview was used to establish whether or not subjects had experienced visual hallucinations among other things; those who reported visual hallucinations were then included in a second interview to obtain details regarding the hallucinations | Unclear |
| Gilmour et al. 2009 | Canada | Cohort | Attendees of low vision clinic with a BCVA ≤20/40 or a reduced visual field of ≤120°; all participants had normal cognition (MMSE ≥22). | 258 | Mean age 80 years (range, 41 to 99 years) | 76% of all patients had AMD; remaining 24% had one or more of the following: diabetic retinopathy, glaucoma, optic nerve dysfunction, corneal diseases, cataracts, or retinitis pigmentosa | Repeated questioning during history taking | Presence of CBS was defined as presence of formed or unformed visual hallucinations with the absence of primary or secondary delusions, full or partial retention of insight into the unreal nature of the hallucinations, and absence of hallucinations in any other sensory modalities |
| Gordon 2016 | Canada | Cross-sectional | First time attendees of a low vision rehabilitation clinic; BCVA and visual field restriction were transformed into a vision code score, which made it impossible to extract detailed BCVA or visual field data | 2,565 | 41% were aged 40 to 80 years, and the remaining 59% were aged 81+ years; 63% were female | 61% had AMD, 12% had glaucoma, 7% had diabetic retinopathy, and 20% had other eye diseases | Presence of CBS was evaluated based on the answers to a specific question regarding CBS: “Many people who come to CNIB tell us that they see things they know are not there. Some see patterns or shapes. Others see images of people or animals. Have you ever experienced this?” | Unclear |
| Jackson et al. 2007 | USA | Cross-sectional | First time attendees of a low vision rehabilitation clinic; of the 225 participants in total, our review focuses on participants with moderate visual acuity or worse (<6/18) | 124 | Mean age 80 years; 70% were female | 63% had AMD; no further data on diagnosis was reported | Presence of CBS was evaluated based on answers to the questions: “Some patients with partial vision who come to the Clinic tell us that they see things that they know are not there. They may see colored shapes or organized patterns or they may even see vivid images of people, animals or flowers.” and “Have you ever experienced this?” | Only reports of formed images were considered hallucinations |
| Marmamula et al. 2019 | India | Cross-sectional | Population-based study in two districts in Telangana, India; all participants underwent eye examination and questionnaires; of the 6,000 participants in total, our review focuses on participants with moderate visual impairment or worse (<6/18); questions were used to exclude participants with cognitive impairment | 774 | All were aged 40 or more; specific demographics of participants with visual impairment was not reported | No specific data for the population of interest in our review was reported; however, the majority had cataract, and the remaining patients had refractive errors or other eye diseases | Presence of CBS was evaluated based on the answers to a specific question regarding CBS: “Have you experienced seeing any imaginary images such as animals, trees, flowers, people etc.?” | Unclear |
| Menon 2005 | United Kingdom | Cohort | Consecutive patients with BCVA of ≤20/200 from one ophthalmic clinic | 48 | Mean 79.1 (SD: 10.9) years (range, 43 to 93 years); 54% were female | 50% had AMD, 31% had glaucoma, 19% had diabetic retinopathy with or without macular edema, and the rest had myopic maculopathies, keratopathies, optic atrophies, optic neuropathies, aphakia, cataract, retinal detachment, and macroaneurism | Presence of CBS was evaluated based on a structured history taking with an initial non-leading question about the existence of symptoms other than visual impairment itself; if a history of visual hallucination was not volunteered on non-leading questions, a leading question was posed; if hallucinations were admitted to, history taking was continued to elucidate different aspects of such hallucinations | Unclear |
| Shiraishi et al. 2004 | Japan | Cross-sectional | Consecutive patients from one ophthalmic clinic; of the 1,000 individuals seen in the outpatient clinic by either an ophthalmologist or an optometrist, 372 were low vision patients (defined as a BCVA of ≤0.3 Snellen); these individuals could be of any age; the authors stratified these 372 individuals into young (n=252) and elderly (n=120); elderly were defined as being >64 years of age; patients were excluded in case of the presence of any psychiatric disorder that can produce visual hallucination | 120 | All individuals in this subset of patients were defined as being >64 years of age | No data | Participants were screened for CBS using the question: “When people suffer from low visual acuity, they sometimes see things and/or other people that are not there. Has such phenomenon ever happened to you?”; in case of a positive answer, the patients were interviewed by a psychiatrist on visual hallucinations to confirm and elaborate on the nature of such visual hallucinations | CBS was defined according to the following criteria: at least one complex visual hallucination within the past 4 weeks; a period between the first and the last hallucination exceeding 4 weeks; full or partial retention of insight into the unreal nature of the hallucinations; absence of hallucinations in other sensory modalities; absence of delusions; the authors did not elaborate whether all or some of these criteria had to be fulfilled to diagnose CBS |
| Tatlipinar et al. 2001 | Turkey | Cross-sectional | Patients were referred to a vision rehabilitation clinic; participants had BCVA in best-seeing eye of ≤20/100 or worse; participants were not known with any psychiatric disorders and were orienteered in time, place, and person | 80 | Mean age 56 years (range, 12 to 88 years); 49% were female | 48% had AMD, 14% had Stargardt’s disease, 10% had diabetic retinopathy, and the rest had glaucoma, retinitis pigmentosa, cone dystrophy, uveitis, optic atrophy, albinism, and CNV secondary to anguloid streaks | Patients were asked in a non-threatening manner if they had ever experienced seeing something that really was not there; it was stressed to the patient that other patients with low vision have similar experiences | Unclear |
| Teunisse et al. 1995 | The Netherlands | Cross-sectional | Attendees from a low vision clinic and an optometrist clinic; of the 600 patients in total, only data from the low vision clinic aged >64 years are included in this review; positive cases underwent MMSE and Geriatric Mental State Schedule | 221 | All were aged >64 years (mean 77.1 years; SD 6.5 years); 67% were female | No specific data for the population of interest in our review was reported; however, the majority of patients had AMD, and the remaining patients had cataract, diabetic retinopathy, glaucoma, or corneal diseases | Presence of CBS was based on a semi-structured interview, where the interviewer afterwards decided whether a subject had “no”, “possible”, or “probable” hallucinations | If the interviewed decided that the hallucinations were either “possible” or “probable”, the patient would have a psychiatrist examination including GMSS and MMSE; the psychiatrist then decided whether the hallucination was CBS |
| Vikucevic & Fitzmaurice 2008 | Australia | Cross-sectional | Consecutive patients with BCVA ≤6/12 from four ophthalmic clinics without any history of psychiatric disorders or use of medication known to cause visual hallucinations, history of hallucinations in other sensory modalities, report of excess alcohol use, or taking illicit or hallucinogenic drugs | 200 | Mean 77.7 (SD 7.4) years (range, 65 to 92 years) | No specific data for the population of interest in our review was reported; however, the majority of patients had AMD, and the remaining patients had diabetic retinopathy, other retinal diseases, or anterior segment pathologies | Participants were screened for CBS using the question: “We had a patient here the other day that had a similar problem with their eyes to yourself. The condition made it difficult to see things and they also noticed that they could see things that were really not there or that other people don’t see. Has this ever happened to you?” | Only reports of complex hallucinations were considered cases of CBS |
AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; BRVO, branch retinal vein occlusion; CBS, Charles Bonnets syndrome; CNIB, Canadian National Institute for the Blind; CNV, choroidal neovascularization; GMSS, Geriatric Mental State Schedule; MMSE, Mini Mental State Examination; SD, standard deviation; USA, United States of America.
Crumbliss et al. (2008) evaluated presence of CBS in consecutive patients presenting for initial evaluation to a visual rehabilitation clinic (23). Of the 12 individuals with visual hallucinations categorized as CBS, six had daily events with CBS hallucinations (23). Elflein et al. (2016) investigated CBS in a visual rehabilitation clinic where patients with visual impairment stratified according to age (≤40 years vs. >40 years), and a control group without visual impairment stratified according to age (≤40 years vs. >40 years) (24). The control group did not experience any visual hallucinations regardless of age (24). The authors found a small but statistically insignificant difference in the prevalence of CBS between young and older patients with visual impairment (24). Gilmour et al. (2009) did not find differences in prevalence of CBS across different diagnoses among attendees of a low vision clinic (25). There was a trend of higher likelihood of CBS with longer duration of the disease (25). Gordon (2016) surveyed new patients of a national low vision institute and found that the risk of CBS correlated with the extent of vision loss and that the underlying ophthalmic diagnosis did not influence the risk of CBS (26). Jackson et al. (2007) evaluated the association between visual parameters and presence of CBS in patients referred for low vision rehabilitation (27). Here, a multiple logistic regression analysis revealed that low contrast sensitivity was the strongest predictor of CBS hallucination after adjusting for best-corrected visual acuity (27). In a population-based screening study, Marmamula et al. (2019) screened 6,000 individuals in 120 clusters of the Khammam and Warangal districts of India (22). Of these, we only considered the 774 individuals for our quantitative review who had moderate visual impairment (<6/18 to 6/60 in best-seeing eye), severe visual impairment (<6/60 to 3/60 in best-seeing eye), and blindness (<3/60 to no light perception in best-seeing eye), although it was unclear if the reported visual acuity was the best-corrected or uncorrected (22). Risk of CBS increased with visual impairment (21). An interesting insight from this study was that 15.6% of individuals with visual impairment due to cataract reported having CBS (22), which is a rate similar to that seen in other reports focused on retinal diseases with affection of central vision (5,6,28). Menon (2005) evaluated presence of CBS among a consecutive range of patients with a best-corrected visual acuity of 20/200 or worse in the better eye, and for comparison recruited an equal number of control individuals of elderly patients with a best-corrected visual acuity of 20/40 or better in their better eye (29). The authors found that 63% of these visually impaired patients admitted to having experienced complex hallucinations, whereas none in the control group had any complex hallucinations (29). Shiraishi et al. (2004) screened for complex hallucinations among 1,000 consecutive Japanese patients seen at an ophthalmic clinic either by an ophthalmologist or an optometrist for various eye diseases (30). Among those defined as low vision (best-corrected visual acuity of 0.3 Snellen or worse in the better eye), 0.8% had CBS (30). The authors then stratified data based on age (≤64 vs. >65 years) and found no significant age-related difference in the prevalence of CBS (30). Tatlipinar et al. (2001) found that approximately one out of three patients referred to low a low vision rehabilitation clinic experienced elementary or complex visual hallucinations irrespective of age (31). Teunisse et al. (1995) found that patients attending a low-vision unit were 11 times more likely to experience CBS compared to those with relative preserved vision in the optometry unit (32). The authors found that a best-corrected visual acuity of 6/18 Snellen or worse in the best-seeing eye was a significant risk factor of CBS (32). No clear association was found to the underlying ophthalmic diagnosis (32). From this study, we only included patients from the low-vision group aged >64 years (n=221) for our quantitative review (32). Vikucevic & Fitzmaurice (2008) screened 200 consecutive patients with best-corrected visual acuity of 6/12 or worse from four ophthalmic clinics and found a high rate of CBS, but no clear association to any specific ophthalmic diagnosis (33).
Risk of bias evaluation of individual studies revealed that most studies clearly defined the data source, eligibility criteria of participants, and described and/or controlled confounding. Time period of study, consecutive recruitment, explanation of exclusions, and any quality assurance for certainty of the employed methods were less consistently declared. Our risk of bias evaluation of individual studies are summarized in Table 2.
| Reference | Defines source | Eligibility criteria | Time period | Consecutive recruitment | Quality assurance | Explains exclusions | Describes confounding |
|---|---|---|---|---|---|---|---|
| Crumbliss et al. 2008 | Yes | No | No | Yes | Yes | Yes | Yes |
| Elflein et al. 2016 | Yes | Yes | Yes | Unclear | Yes | NR | Yes |
| Gilmour et al. 2009 | Yes | Yes | No | No | No | Yes | No |
| Gordon 2016 | Yes | Yes | Yes | Unclear | No | Yes | Yes |
| Jackson et al. 2007 | Yes | No | Yes | Yes | No | NR | Yes |
| Marmamula et al. 2019 | Yes | Yes | Yes | Yes | No | No | Yes |
| Menon 2005 | Yes | Yes | Yes | Yes | Yes | NR | Yes |
| Shiraishi et al. 2004 | Yes | Unclear | No | Yes | Yes | NR | No |
| Tatlipinar et al. 2001 | Yes | Yes | Yes | Unclear | No | NR | Yes |
| Teunisse et al. 1995 | Yes | Unclear | No | Yes | Yes | NR | No |
| Vikucevic & Fitzmaurice 2008 | Unclear | Yes | No | Yes | Yes | Yes | Yes |
In these cases, authors do not explain any reason for exclusion because there are no exclusions. Studies are assessed on relevant items from the Agency for Healthcare Research and Quality checklist. Defines source: defines the source of information. Eligibility criteria: lists inclusion and exclusion criteria or refers to previous publications. Time period: indicates time period used for identifying participants. Consecutive recruitment: indicates whether or not subjects were consecutively recruited for the study. Quality assurance: describes any assessments undertaken for quality assurance purposes. Explains exclusions: explains any patient exclusions from analysis. Describes confounding: describes how confounding was assessed and/or controlled. NR, not relevant.
The random-effects pooled prevalence of CBS in low vision patients was 19.7% (95% confidence interval: 13.8% to 26.4%) (Figure 2). Considerable heterogeneity across studies was present, which was quantified as an I2 at 95%. The Funnel plot did not suggest a strong presence of publication bias (Figure S1). The sensitivity analysis showed certain robustness in the summary prevalence estimate, since excluding studies by turn only lead to minor deviation from the overall estimate with prevalence estimates ranging from 16.9% to 22.5% (Table S1).
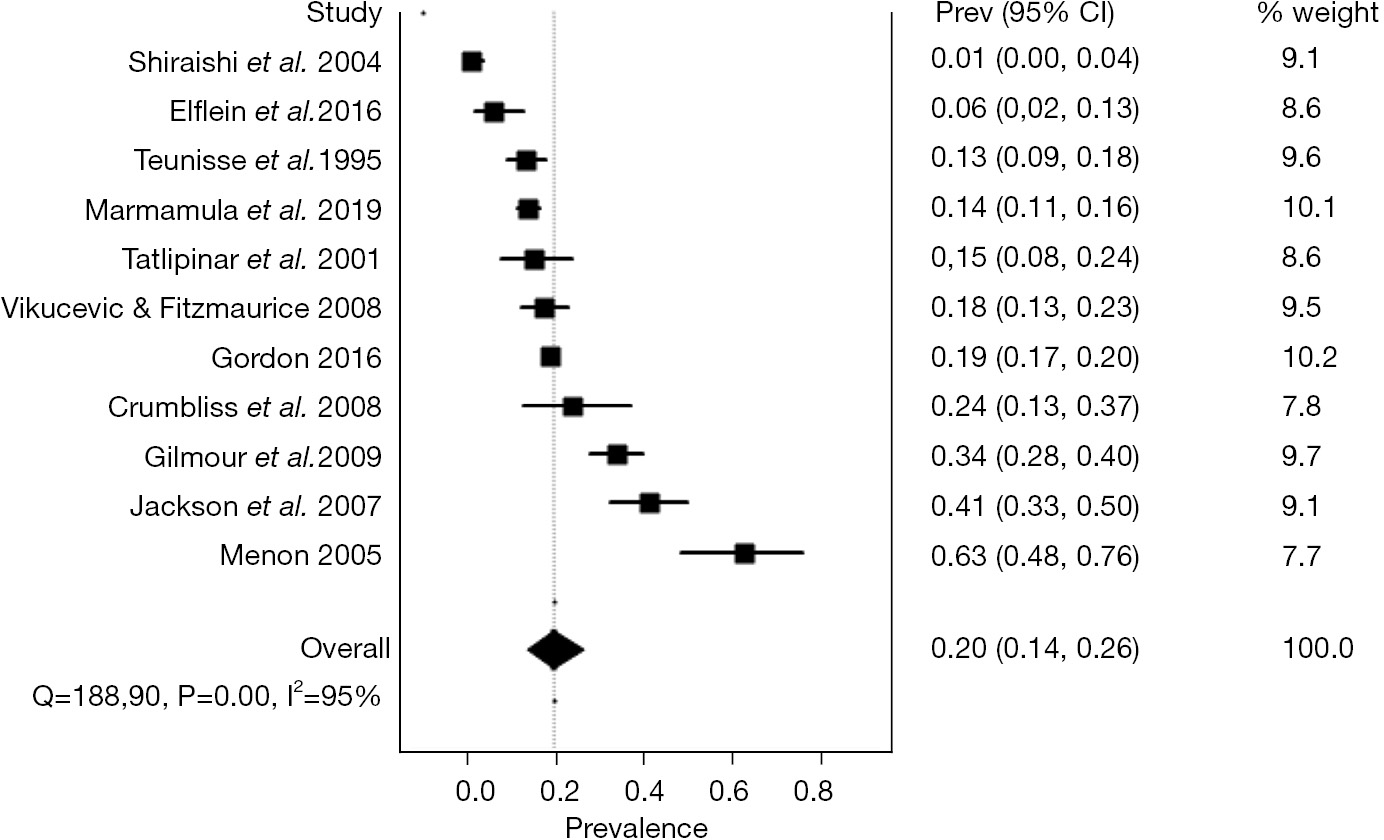
In this systematic review and meta-analysis of 11 studies on patients with low vision, we find that the prevalence of CBS can be summarized to 19.7%. In other words, one in five patients with low vision have CBS. This is a simple but important message and should be kept in mind when dealing with patients with low vision. Furthermore, this estimate also states that CBS is prevalent at a rate which necessitates important attention from healthcare stakeholders, justify attention on planning and allocating resources to CBS, and suggests that a business case should be possible for development of therapy or otherwise medical technology to help these patients. Considering that the majority of cases can be dealt with through information, our findings also call for the need for greater awareness on this condition (28,34).
The currently best estimate on the global prevalence of visual impairment and its future forecast is made by the Vision Loss Expert Group of the Global Burden of Disease Study (35). Available estimates for moderate and severe visual impairment (defined as <6/18 to 3/60) and blindness (defined as <3/60 or less than 10° visual field around central fixation), in individuals aged 50 years or more, can be extracted for geographical regions as defined by the WHO. We used these numbers and our estimate of 19.7% having CBS to calculate number of patients with CBS in geographically defined areas (Table 3). To our best knowledge, these estimates constitute the World’s first reliable estimate on the number of patients with CBS. Among 239 million individuals with moderate visual impairment or worse, we estimate that the global number of patients with CBS is approximately 47.2 million.
| Geographical area | Individuals with MSVI (in 1000s) | Individuals with blindness (in 1000s) | Total number of individuals with MSVI or blindness (in 1000s) | Estimate of individuals with CBS (in 1000s) |
|---|---|---|---|---|
| Andean Latin America | 1,620 | 275 | 1,895 | 373 |
| Australasia | 472 | 58 | 530 | 104 |
| Caribbean | 967 | 205 | 1,172 | 231 |
| Central Asia | 1,930 | 222 | 2,152 | 424 |
| Central Europe | 3,270 | 279 | 3,549 | 699 |
| Central Latin America | 5,760 | 970 | 6,730 | 1,326 |
| Central Sub-Saharan Africa | 1,030 | 167 | 1,197 | 236 |
| East Asia | 44,900 | 7,260 | 52,160 | 10,276 |
| Eastern Europe | 8,940 | 682 | 9,622 | 1,896 |
| Eastern Sub-Saharan Africa | 3,600 | 1,200 | 4,800 | 946 |
| High-income Asia Pacific | 4,080 | 450 | 4,530 | 892 |
| High-income North America | 4,750 | 591 | 5,341 | 1,052 |
| North Africa and Middle East | 12,000 | 2,350 | 14,350 | 2,827 |
| Oceania | 222 | 23 | 245 | 48 |
| South Asia | 68,800 | 9,580 | 78,380 | 15,441 |
| Southeast Asia | 19,800 | 4,550 | 24,350 | 4,797 |
| Southern Latin-America | 1,240 | 125 | 1,365 | 269 |
| Southern Sub-Saharan Africa | 870 | 353 | 1,223 | 241 |
| Tropical Latin-America | 5,710 | 1,430 | 7,140 | 1,407 |
| Western Europe | 11,100 | 1,350 | 12,450 | 2,453 |
| Western Sub-Saharan Africa | 5,530 | 1,490 | 7,020 | 1,383 |
| Global | 206,000 | 33,600 | 239,600 | 47,201 |
The numbers of individuals with MSVI, blindness, and with CBS are all listed in estimates from year 2020 and in 1000s of individuals. The numbers of individuals with MSVI and blindness is courtesy of the Vision Loss Expert Group of the Global Burden of Disease Study (32). CBS, Charles Bonnet syndrome; MSVI, moderate-to-severe visual impairment.
It is important to acknowledge the limitations of our study when interpreting its results. Firstly, we evaluate patients who are referred to a low vision clinic or visual rehabilitation clinic. There may be a bias in that patients may be more likely to be referred or turn up to consultations if they are relatively more affected by their disease, which one can speculate could be reflective of a higher prevalence of CBS. Secondly, the majority of cases had AMD, which also reflects the leading reason for visual impairment in elders in developed countries. The high prevalence of CBS in AMD has been extensively documented (5). However, the picture may differ in developing countries where other eye conditions are the leading reason for visual impairment, which introduces an uncertainty on the estimates, at least for developing countries where we only had one study to provide data (22). Third, pediatric cases of CBS and CBS related to inherited causes of visual impairment are not considered in our review. Emerging evidence suggest that such cases may be underestimated (36,37). Therefore, our estimates may underestimate the total number of CBS. Finally, there are no validated questionnaires or gold standards for diagnosis of CBS, and the approach for diagnosis differed across studies. To further complicate the matters, sociocultural aspects may also potentially influence on the response rate through different methodological approaches. These uncertainties also contribute as a limitation to our summary estimate.
In conclusion, we find that CBS is relatively common in patients with low vision at a rate of approximately one in five patients. Extrapolating this estimate number of individuals with visual impairment highlight that CBS is no rare condition and that CBS warrants further attention from ophthalmologists as well as healthcare stakeholders.