Background and Objective: Subthreshold laser therapy has emerged as a therapeutic alternative to traditional laser photocoagulation for certain ophthalmic diseases including central serous chorioretinopathy (CSCR), diabetic macular edema (DME), macular edema secondary to branch retinal vein occlusion (BRVO), and age-related macular degeneration (AMD). The objective of this paper is to review and discuss the clinical applications of subthreshold laser and the mechanisms of different subthreshold laser techniques including subthreshold micropulse laser (SMPL), selective retina therapy (SRT), subthreshold nanosecond laser (SNL), endpoint management (EpM), and transpupillary thermotherapy (TTT).
Methods: A narrative review of English literature and publicly available information published before November 2021 from literature databases and computerized texts. We discuss the currently available subthreshold laser systems and the advancements made to perform different subthreshold laser techniques for various ophthalmic diseases. We highlight various clinical studies and therapeutic techniques that have been conducted to further understand the effectiveness of subthreshold laser in the clinical setting. We conclude the article by covering emerging subthreshold laser systems that are currently being developed for future clinical use. The PubMed database was utilized for peer-reviewed articles and pertinent information on subthreshold systems was cited from publicly available online websites covering specific systems.
Key Content and Findings: Various subthreshold laser systems have been developed to treat certain retinal diseases. Several systems are currently in development for future clinical applications.
Conclusions: While conventional laser photocoagulation has been effective in treating various retinal diseases, subthreshold laser systems aim to provide a therapeutic effect without visible signs of damage to the underlying tissue. This technology may be particularly effective in treating macular disorders. Further clinical studies are needed to evaluate their role in the management of retinal diseases.
Traditional or conventional laser photocoagulation became the treatment of choice for many retinal diseases including proliferative diabetic retinopathy and diabetic macular edema (DME). The technology was developed in the 1950s through the 1970s. Laser energy effects on ocular tissues depend upon the wavelength, pulse duration of the laser light, laser power and the absorption characteristics of the tissue in question, which is largely determined by the pigments contained within it. During conventional laser photocoagulation a continuous wave of energy is delivered to the target tissue throughout the entire duration of the laser pulse. Light energy is converted into heat energy if the wavelength coincides with the absorption spectrum of the tissue pigment on which it falls. Pigmented tissues that absorb laser energy in the macular area include the retinal pigment epithelium (RPE), choroid, hemoglobin within red blood cells and the xanthophyll pigment. These pigments absorb the laser energy and convert it to heat which leads to an increase in tissue temperature. Depending on the duration and magnitude of the laser pulse, the heat wave will expand laterally and vertically and often results in collateral damage to adjacent tissues. In the past, visible burns were believed to be necessary for a successful treatment. Thus the traditional endpoint was the achievement of a visible (threshold) grayish burn (1). The exact mechanisms underlying conventional laser photocoagulation currently remains under investigation, but studies have shown local increases in preretinal or intraretinal oxygen tension in regions overlying photocoagulation (2-4). Additionally, laser photocoagulation been shown to reduce vascular endothelial growth factor (VEGF) production and improve the outcomes in ischemic retinopathies (5). However, this destructive treatment may not be ideal at the macula as laser-induced scars may create scotomas and complications of traditional laser photocoagulation include subretinal neovascular membranes and subretinal fibrosis (6).
For the past 30 years, there has been ongoing development and increased clinical use of an alternative treatment to conventional laser treatment known as subthreshold laser therapy (7,8). Subthreshold laser refers to a spectrum of photocoagulation techniques that provides a therapeutic effect in treating retinal or macular diseases while avoiding damaging laser scars in the treated area (9). Several lines of evidence suggest that threshold burns may not be required for the therapeutic benefits of photocoagulation. Thus, subthreshold laser has been developed to preserve the RPE while effectively treating the underlying macular pathology (8,10). On a molecular level, subthreshold laser modulates heat-shock protein expression in the RPE and regulates cytokine expression, leading to therapeutics effects without the permanent destruction to the retinal tissue (6,11). In contrast conventional laser photocoagulation leads to coagulative necrosis of the target tissue. The balance point between therapeutic effect and non-lethal lasering has been rigorously studied and the utilization of this technology continues to advance for clinical practice. In this review, we discuss the mechanisms behind subthreshold laser therapy as well as the current and evolving clinical applications for this technology in retinal diseases. We also review the similarities and differences between current subthreshold laser systems commercially available for clinical use. We conclude this paper with a discussion of the future directions for subthreshold laser technology. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-21-46/rc).
In this narrative review, we reviewed relevant literature pertaining to subthreshold laser systems in the English language. We included peer-reviewed articles reporting clinical research and basic science research. To include pertinent information about commercially available subthreshold laser systems and relevant history, we also included information from commercially available web pages that have been cited accordingly. The end date of peer-reviewed articles included is October 2021. For peer-reviewed literature articles, we included the followings terms for the literature search in the context of subthreshold laser systems for retinal diseases: “Subthreshold Micropulse Laser”, “Micropulse Diode Laser”, “Photocoagulation”, “Selective Retina Therapy”, “Retina”, “Subthreshold Nanosecond Laser”, “Endpoint Management”, “Transpupillary Thermotherapy”, “Laser Endpoint”, “Micropulse”, Age-related Macular Degeneration”, “Central Serous Chorioretinopathy”, “Diabetic Macular Edema”, “Retinal Vein Occlusion” (Table 1).
| Items | Specification |
|---|---|
| Date of search | Narrative review search timeline: April 2021–November 2021 |
| Databases and other sources searched | PubMed and pertinent publicly available web pages regarding commercially available subthreshold laser systems and relevant history |
| Search terms used (including MeSH and free text search terms and filters). Note: please use an independent supplement table to present detailed search strategy of one database as an example | For peer-reviewed literature articles, we included the followings terms for the literature search in the context of subthreshold laser systems for retinal diseases: “Subthreshold Micropulse Laser”, “Micropulse Diode Laser”, “Photocoagulation”, “Selective Retina Therapy”, “Retina”, “Subthreshold Nanosecond Laser”, “Endpoint Management”, “Transpupillary Thermotherapy”, “Laser Endpoint”, “Micropulse”, Age-related Macular Degeneration”, “Central Serous Chorioretinopathy”, “Diabetic Macular Edema”, “Retinal Vein Occlusion” |
| Timeframe | Till November 2021 |
| Inclusion and exclusion criteria | Relevant literature pertaining to subthreshold laser systems in the English language |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | All authors |
On July 9, 1945, there was a solar eclipse that caused retinal injury to individuals who gazed for too long (12). Dr. Meyer-Schwickerath took care of a patient that had sustained injury by the eclipse and noticed burns on the macula. Dr. Meyer-Schwickerath theorized that light-induced burns may have a therapeutic effect on the retinal. He developed and continued to refine this technique, and in 1949, he performed the first light coagulation procedure on a patient with imminent retinal detachment (12). Dr. Meyer-Schwickerath published his groundbreaking report (13) in 1954 on the therapeutic use of light-induced burns on the retina which became a revolutionary landmark for laser photocoagulation in ophthalmology. In 1956, Dr. Meyer-Schwickerath and Dr. Littmann from Zeiss Laboratories developed the xenon-arc photocoagulator (14,15). The evidence for the therapeutic effects of photocoagulation were so strong, that a randomized study of photocoagulation for diabetic retinopathy was halted because it would have been detrimental to withhold treatment from the control group (12). It is important to note that the xenon-arc photocoagulator is not classified as a laser. In addition to the xenon-arc photocoagulator, ruby laser (694 nm wavelength), argon blue laser (488 nm wavelength), and argon green laser (514 nm wavelength) have been developed and used in clinical practice to deliver the full laser photocoagulation (15). The technology has revolutionized ophthalmic care; however, the need to spare retinal tissue in certain diseases while providing therapeutic effects of photocoagulation led to the development and refinement of subthreshold laser techniques.
There are five distinct types of subthreshold laser. These five are subthreshold micropulse laser (SMPL), selective retina therapy (SRT), subthreshold nanosecond laser (SNL), endpoint management (EpM), and transpupillary thermotherapy (TTT) (8,15-18) (Table 2). While all five laser techniques have the end goal of minimizing damage to the targeted tissue, there are fundamental differences in their underlying mechanisms to differentiate their treatment effect. From a clinical perspective, it is critical to understand the fundamental mechanism in order to identify which modality is best suited for specific pathologies. In this section, we describe each subthreshold laser type, its mechanism and clinical applications in retinal disease, as well as the corresponding commercially available systems for clinical use.
| Subthreshold laser type | Mechanism (operation mode of laser emission) | Utilization in retinal disease |
|---|---|---|
| Subthreshold micropulse laser (SMPL) also known as subliminal laser | “On” and “Off” time duty cycle, typically around 50–1000 microseconds | CSCR, DME, macular edema secondary to BRVO (15,17,19) |
| Selective retina therapy (SRT) | Microsecond laser pulses to selectively damage specific RPE cells while sparing surrounding tissue | CSCR, DME, diabetic retinopathy |
| Subthreshold nanosecond laser (SNL) | Nanosecond laser pulses | DME, AMD (20,21) |
| Endpoint management (EpM) | Pattern scanning with short pulses | CSCR, DME, non-proliferative diabetic retinopathy, macular telangiectasia type 2 (22-25) |
| Transpupillary thermotherapy (TTT) | Extended duration laser with decreased irradiance | Neoplastic choroidal disease (26-28), CSCR (29-31) |
CSCR, central serous chorioretinopathy; DME, diabetic macular edema; BRVO, branch retinal vein occlusion; AMD, age-related macular edema.
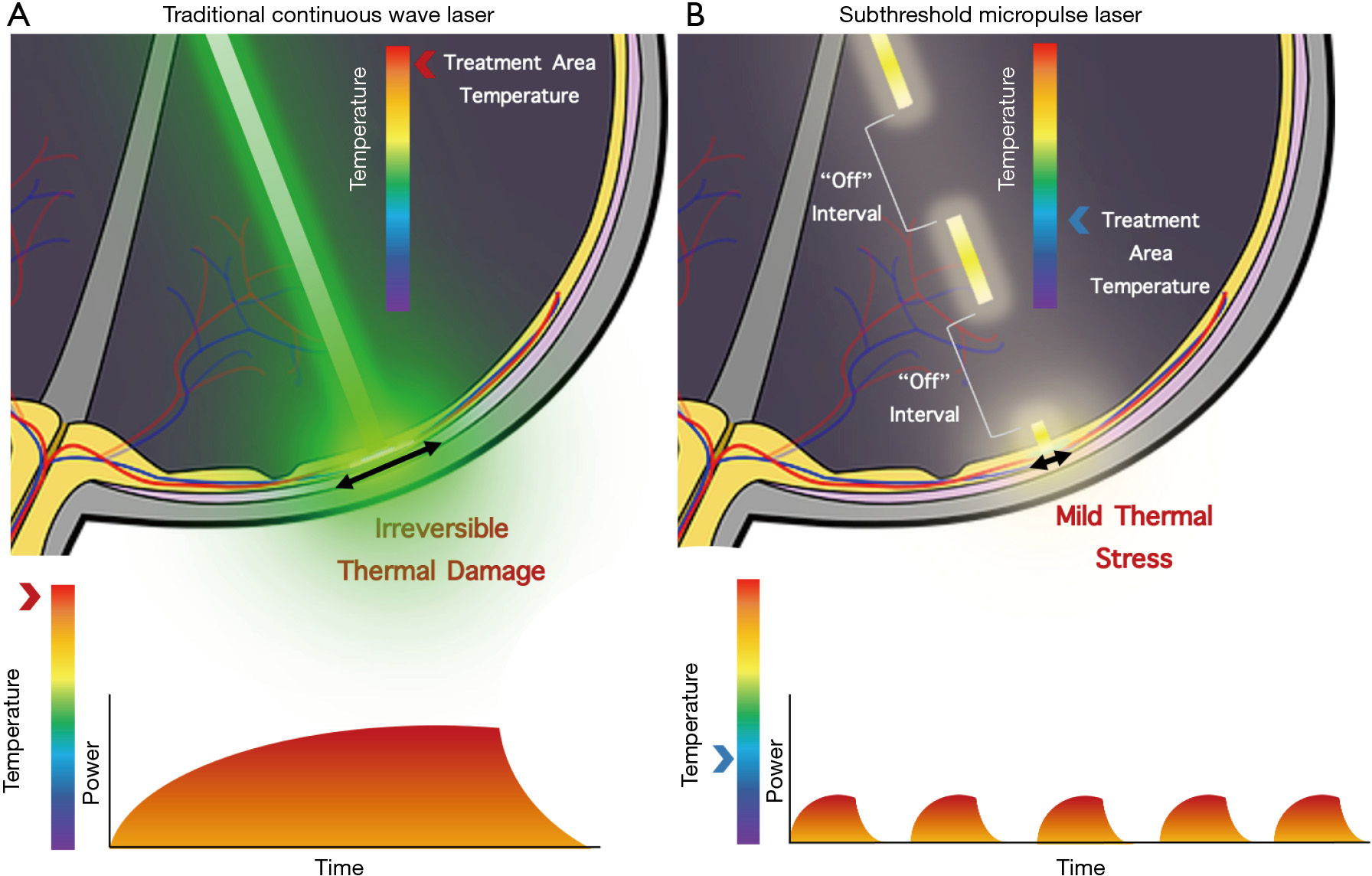
SMPL is a widely utilized subthreshold laser technique with many aliases including microsecond pulsing, subthreshold diode micropulse laser, MicroPulse, SubLiminal, and SmartPulse. SMPL achieves subthreshold effects by delivering laser energy via repetitive short pulses separated by periods where the laser does not fire instead of a continuous wave (15,17,32). Each pulse typically varies in duration between 100–300 microseconds, although some systems allow adjustments between 50–1,000 microseconds (8). This duration is referred to as the “On” time. Between the “On” there are “Off” times where the laser is no longer applied to the tissue. The ratio of “On” to “Off” times is known as the duty cycle, which is reported as a percentage, typically set to 5%, 10%, or 15% for subthreshold laser (8). A duty cycle below 100% and the use of “Off” times is what distinguishes SMPL from traditional lasers and allows the tissue time to cool down, preventing laser-induced retinal damage (Figure 1). Although initial experimentation was done with 810 nm wavelength lasers, 577 and 532 nm wavelength lasers can also be used with SMPL and have become more commonplace recently (33,34). Chhablani et al. studied the safety of various parameters for SMPL. In this retrospective study, varying parameters including duty cycles, spot sizes, and titration in SMPL led to no damage to the tissue. The authors conclude the manuscript with the necessity for further prospective studies with SMPL (35).
Developed in 1990, the micropulse diode laser (MPL) was a novel concept and one of the first technologies to have subthreshold capabilities (36). However, it was not until 2000 when a pilot study with a “low-intensity” MPL treatment was begun, that the technology was used for its subthreshold effects (37). This treatment, now termed SMPL, challenged the notion that threshold burns are necessary to effectively treat DME. Since then, micropulse technology has been used and tested in clinical settings, with applications spanning from retinal diseases to glaucoma.
The exact mechanism of action of ST-MPL remains unclear (17). Sublethal photothermal stimulation with SMPL was shown to stimulate RPE to repair of the inner blood retinal barrier (iBRB), alter the genes in the wound healing process and increase the expression of heat shock proteins (HSPs) without destroying of the photoreceptors in an in-vitro study (6). A recent mice study demonstrated that SMPL restores oxidant/antioxidant balance in the retinal layers and modulates apoptosis within cells (38). When mouse RPE cells are treated with ST-MPL, the protein and mRNA expression level of pro-angiogenic cytokines and growth factors such as VEGF-A, TGF-? and bFGF, were downregulated. Midena et al. compared the anterior chamber cytokine profile of eyes with DME prior to ST-MPL and 9 months after ST-MPL, they found a significant reduction in inflammatory cytokines primarily produced from retinal microglia including MIP1α, RANTES, FasL, and VEGF. Microglia serve as the immune cells in the retina. The authors concluded the study suggesting that ST-MPL may deactivate microglial cell activation, thus decreasing the retinal inflammatory response in DME (39). Midena et al. observed in another report that using ST-MPL with untreated DME led to reduction of glial fibrillary acidic protein (GFAP) and inwardly rectifying potassium (Kir) 4.1 channels in the aqueous humor. GFAP and Kir 4.1 have been identified as biomarkers of Müller cell activation, thus, providing additional insight into the mechanism of ST-MPL (40). Additional molecular studies may aid in further elucidating the mechanism of ST-MPL’s therapeutic effect.
Various clinical applications for SMPL have been explored including DME, CSCR, proliferative diabetic retinopathy, BRVO, AMD and glaucoma (15,17,19). SMPL is a form of subthreshold laser application that is used with different laser wavelengths including 532, 532, and 810 nm; 577 and 810 nm are frequently utilized in SMPL therapy (41). Across two studies for CSCR, pigmentary changes at the RPE were seen in six patients (7,42). However, there were no visual implications, scar formation, or visible laser burns. A prospective clinical study using SMPL for persistent CSCR lasting longer than 3 months observed an improvement in visual acuity as well as reduction in central macular thickness (43). In the treatment of DME, there was scar formation in a few eyes across multiple studies, albeit only when treated at a 15% duty cycle (8). Uniquely, SMPL applied to the fovea to treat fovea involving DME had no evidence of laser injury to the RPE or neurosensory retina by any imaging method (44). It should also be noted that laser titration may have the risk of overtreating the area of interest (19). Current clinical studies are being conducted to study the effects of SMPL panmacular treatment verses minimal treatment in CSCR (45). The observations from this clinical trial may provide additional insight into the optimal protocol in treating CSCR with SMPL. In 2016, a prospective randomized clinical trial comparing SMPL and conventional laser in DME observed increased reduction in central macular thickness and central macular volume in SMPL compared to traditional photocoagulation (46). Currently, may provide further insight into its utility for specific retinal conditions.
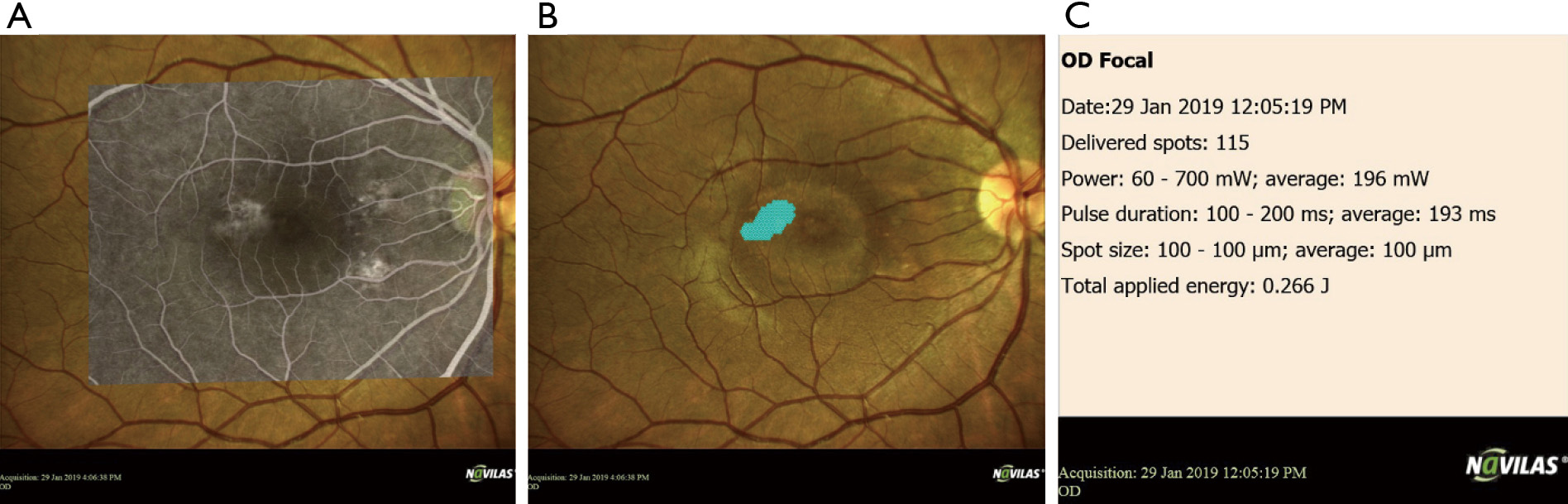
One challenge that SMPL faces is a lack of standardized treatment parameters and titration protocols. Duty cycles, wavelengths, and laser power differ between studies and clinicians. Additionally, there is a potentially high risk of undertreatment due to the inability to visualize visible burns (8). However, these limitations are being addressed by advanced navigated laser techniques that allow for effective pre-planning, recorded treatment, and increased accuracy (47) (Figure 2).
EpM, an algorithm introduced in 2014 by Topcon Medical Systems, uses the computational model of retinal photothermal damage to minimize tissue damage. The protocol adjusts the laser power and duration to achieve subthreshold effects by analyzing the visible threshold. This technique was introduced with the PASCAL Streamline 577 system, which provided a solid-state continuous wave laser coupled into a scanning system. The configurations for the initial experiment were 10–100 millisecond pulses and 100–400 micrometer spot sizes (18). The EpM software distinguishes itself as it uses a computational model of retinal heating in order to map the laser pulse energy to the expected tissue effect. By doing so, the theoretical exact laser pulse energy can be determined from the desired tissue effect (18).
EpM aims to reduce retinal damage by augmenting the power and duration of a continuous wave laser. Retinal damage can occur due to thermal denaturation of tissue as a result of pulse durations greater than 50 microseconds. This damage can be described using the Arrhenius law and first-order reaction kinetics (18). The decrease in the concentration of the critical molecular component corresponds to retinal damage, and by extension, the rate of this decrease D(t) changes with temperature T(t). By taking the integral of this relationship and normalizing to unity, the criterion for cell viability may be determined. Arrhenius values below 0.1 are generally subtherapeutic, between 0.1 and 1 are subthreshold, and much greater than 1 are suprathreshold (18,22). By using this information and a standardized titration dose, the EpM software maps calculated Arrhenius values to linear steps in pulse energy.
For normalizing to occur, the EpM algorithm requires a titration protocol. And 100% energy level, corresponding to an Arrhenius value of 1,000 for 20 millisecond (22), is defined as the laser pulse power required to cause a minimally visible retinal lesion endpoint. This titration burn is conducted outside the arcades. The power starts low and increases until a visible burn is observed after 3 seconds, which defines the 100% energy level. After titration, laser energies used for retinal treatment are defined as a percentage of this 100% energy level, with each percentage corresponding to a unique pulse duration and fractional power (18). The algorithm is set so a 20% increase in the energy level corresponds to a 10-fold increase in the Arrhenius integral (22).
By adjusting the energy levels, different treatment effects may be seen. The reported effects are from burns applied to the retina in rabbits. For values greater than 100%, there is damage to the inner and outer retina and potentially the retinal nerve fiber layer. At 100% energy, the burns may be visible in fluorescein angiography (FA) and optical coherence tomography (OCT), with the absence of photoreceptors in the middle of the burn and restored RPE monolayer after 1 week. For burns between 50–75% energy, there is no observable change ophthalmoscopically, but there are signs of damage via OCT and FA. There is restoration of the RPE monolayer after 3 days. For 30% energy burns, there are no visible damages observed via OCT or FA, and tissue damage is limited to a single central RPE cell. While the 30% energy level is considered the safest and is the recommended treatment, any energy level below 100% qualifies as subthreshold (18).
The theorized underlying mechanism of EpM involves activation of HSPs by appropriate thermodynamic stimulation. Mouse models indicated that Arrhenius values greater than 0.1 led to HSP expression (48). Therefore, the ideal therapeutic window is between the Arrhenius values of 0.1 and 1, to allow for proper activations of HSP without RPE damage (22). This corresponds to pulse energies between 20% and 40% for 20 millisecond pulses.
So far, the efficacy of EpM has been explored for the treatment of CSCR, DME, non-proliferative diabetic retinopathy, and macular telangiectasia type 2 (22-25). In a prospective, nonrandomized, interventional case series, 21 eyes from 20 chronic CSCR patients were treated with EpM at 30% (22). In these eyes, central macular thickness was reduced on average from 362 to 283 micrometers (P=0.001), and complete resolution of subretinal fluid was observed in 81% of patients. No retinal damage was observed by clinical observation, OCT, fundus autofluorescence, or FA in a 1-year follow-up (22). In the same study, 10 eyes from 5 patients with MacTel type 2 undergoing EpM treatment were followed. Contrary to the natural thinning of central macular thickness observed in MacTel, central macular thickness was stabilized from 246 to 241 micrometers (P=0.48). Inner and outer retinal lacunae significantly decreased in 80% of eyes (22).
In a prospective, single-center, paired randomized controlled trial, 56 eyes of 28 patients with bilateral, symmetric, severe nonproliferative diabetic retinopathy (NPDR) were followed (24). One eye from each patient was randomly assigned to receive EpM, and the other was assigned to receive traditional panretinal photocoagulation (PRP). No statistically significant difference was found between the two groups at a 12-month follow-up with regards to best corrected visual acuity (BCVA), central foveal thickness (CFT), and progression to proliferative diabetic retinopathy (24). In addition, a recent study has shown that the EpM algorithm can be approximated by conventional laser systems, widely increasing the usability of the concept (49). In conclusion, EpM shows promise in treating CSCR, DME, NPDR, and MacTel through a novel subthreshold modality. Additional prospective studies will be needed to further understand its effectiveness and longitudinal outcomes in treating these retinal diseases.
SRT uses short pulses on the order of microseconds to target RPE cells while sparing surrounding tissues. The specific targeting of RPE cells prevents long term damage, scarring, and scotoma, while restoring function of photoreceptors or healthy RPE cells. SRT is accomplished by using high energy pulses with very short pulse durations (<5 microseconds). A green wavelength, such as 514, 527, or 532 nm, is typically used to target melanosomes in RPE cells (36,50,51). Current systems utilize a Q-switched Nd:YLF laser with a wavelength of 527 nm, a pulse duration of 1.7 microseconds, a repetition rate of 100 Hz, and a maximum of 15 pulses in a burst (17). However, prototypes in the past decade have used pulse durations from 0.2 to 33 microseconds and repetition rates from 100 to 500 Hz (51). Despite its development in the 1990s, only one system is available commercially, the R:GEN from Lutronic Vision. Accordingly, information on SRT use is relatively low compared to SMPL in subthreshold laser clinical treatment for retinal diseases.
The underlying mechanisms of SRTs involves selective targeting of the RPE cell layer. RPE plays a vital role in maintaining visual function, malfunction of the RPE cells is correlated with many retinal diseases including CSCR and age-related macular degeneration (AMD). Eliminating damaged RPE layers may allow successful regeneration by wound healing, which would restore function to the tissue (52). However, the adjacent neural retina and choroid tissues are highly sensitive, so preventing damage to these regions is desirable. This specific need is what led to the development of SRT, which aims to selectively destroy damaged RPE cells and stimulate wound healing, a process known as “retinal rejuvenation” (17,51).
For this process, SRT causes the formation of transient microbubbles around targeted melanosomes. These bubbles increase cell volume, which leads to mechanical disruption of the RPE cells (53-55). To prevent spread of heat and damage to adjacent cells, the heating needs to occur below the RPE’s thermal confinement time, which is defined as the maximum temperature rise to 36% (51). To achieve this over a 3 micrometer sheet, the pulse duration threshold is 30 microseconds (36). Therefore, using heating times far below this threshold should yield desired effects. Unfortunately, according to the Arrhenius law, to reach an endpoint that causes retinal denaturation, a 30 microsecond pulse requires temperatures that exceed 100 degrees Celsius (56). To overcome this challenge, multiple pulses may be used to reach the endpoint, which is possible since thermal damage is accumulative. Importantly, pulses must be spread appropriately to allow the tissue to cool between pulses and prevent background heating by thermal diffusion (51). For these reasons, SRT typically involves a short pulse duration shorter than 5 microseconds with a repetition rate around 100 Hz to allow for proper thermal cooling.
In addition to microbubble formation, SRT may restore function by altering cytokine release from the RPE. Three days following SRT therapy, RPE VEFG downregulation and upregulation of pigment epithelium derived factor (PEDF) occurs (57). Additionally, the activation of matrix metalloproteinases (MMPs) and HSP 70 may play a positive role in increasing transmembrane molecular exchange (58). This change in the membrane matrix along with the establishment of a new RPE cell layer through wound healing may explain the therapeutic effects of SRT.
SRT has been tested clinically for a variety of applications including DME, CSCR, and intermediate AMD (51). A prospective clinical study with persistent CSCR observed complete subretinal fluid resolution in 60% of eyes after 12 weeks of SRT treatment (59). In DME, SRT was observed to decrease maximum macular thickness as well as increase mean macular sensitivity (60). One of the primary challenges facing clinical SRT treatment is the lack of immediate visual feedback leading to undertreatment. Since the modality is subthreshold, visible changes in the retina are unobservable. Complicating this problem further are the interpersonal and intrapersonal differences in the amount of energy required for desired effects in the RPE (61,62). Even if titration is done, confirmation of treatment is a challenge. Currently, FA is used in order to visualize treatment effects (63). To resolve these limitations, technology regarding detection of endpoint and automated dosing control have emerged. Microbubble formation has been used as the standard to detect successful SRT treatment (53). Optoacoustics, light reflection, small bandwidth interferometry, and broad bandwidth interferometry-optical coherence tomography (OCT) can each be used to detect microbubble formation (51). Proper detection can be used with a ramp mode, which increases energy levels upon successive pulses within a burst, to quickly interrupt treatment once the endpoint has been reached. Additional, artificial convolutional neuronal networks on OCT signals can be used to improve the accuracy of detection (64). Another solution, the Heidelberg SPECTRALIS Centaurus fully integrates SD-OCT technology to an SRT laser platform to provide OCT-based dosing control, therapy planning, and documentation (51).
SNL use ultra-short pulses, on the order of a few nanoseconds, to specifically target RPE cells and spare surrounding tissues. Similar to SRT, a green wavelength is used to target melanosomes (36,50). Prototyping and testing has been conducted via AlphaRET’s 2RT laser, which is a 532 nm Q-switched YAG laser with a 3-nanosecond pulse duration and 400 micrometer spot size and a speckled-beam profile (65).
The hypothesized mechanism of SNL is similar to that of SRT. The targeted elimination of RPE through microbubble formation leads to “retinal rejuvenation” as surrounding healthy RPE fills the lasered tissue (54,66). Additionally, the improvement of the Bruch’s membrane seen after SNL may be due to cytokine release and increased secretion of MPPs (67,68). A combination of these two factors is thought to cause the therapeutic effects of SNL.
SNL shares the many same challenges found with SRT, including difficulty in assessing treatment without visual feedback (51). However, there have not been as many solutions to address this need in the SNL space. Furthermore, in a study done using an 8-nanosecond laser, there was an increased risk of retinal disruption and retinal hemorrhage due to the increased peak temperatures when using SNL as opposed to SRT (69). The RPE-melanosomes that are targeted become more explosive as the pulses become shorter, which can lead to mechanical damage. This results in a narrower therapeutic window compared to traditional SRT in the microsecond range. Fortunately, this window can be widened, albeit not as wide as SRT, by using 10 separate pulses instead of a single pulse (67).
SNL has been tested for clinical use for both DME and AMD. In a randomized, non-inferiority trial, 31 patients with DME were treated, 20 eyes with SNL and 18 eyes with conventional laser (70). The study found that retinal thickness reduction in SNL was not unambiguously non-inferior to conventional laser. However, visual acuity met non-inferior requirements (70). In another study, 23 patients with 38 eyes diagnosed with DME were treated with SNL (20). There was a significant improvement in BCVA, but no significant change in central macular change. This study indicated a beneficial effect from SNL treatment without many of the side effects of conventional laser (20). However, further studies and a randomized control trial must be conducted to confirm SNL treatment effects for DME.
For the treatment of AMD, the multi-center LEAD Trial assessed the efficacy of SNL treatment on the progression of intermediate AMD (iAMD) to late AMD (21,65,71). In the 36-month randomized, sham-controlled trial, 292 patients with bilateral large drusen, defined as having iAMD, received either SNL or sham treatment and were followed on progression to late AMD. Progression was not significantly slowed with SNL treatment, however during post-hoc analysis, progression was slowed for patients without coexistent reticular pseudodrusen (RPD) whereas eyes with RPD experienced a more rapid progression to late AMD than sham treated eyes (21).
An additional 24-month observational extension was done following the LEAD trial which confirmed that SNL treatment did not significantly reduce the overall rate of progression to late AMD unless considering only patients without coexistent RPD (72). Recently, a cohort study was performed where 64 eyes treated with SNL were compared to 77 untreated, control eyes with early or iAMD (73). There was a significant reduction in the area and amount of drusen after 6 months for SNL treatment. However, there was no significant difference in visual acuity for both groups (73). As a result, future studies should still be conducted to establish the efficacy of SNL treatment for AMD progression in eyes with iAMD without RPD.
SNL treatment for acute and chronic CSCR achieves restoration of macular anatomy and functional improvement without causing alteration of the RPE (74).
TTT is a subthreshold laser technique utilizing a long-pulse, low-irradiance, and infrared photocoagulation laser (75) (Figure 2). Contrary to the previous techniques mentioned, TTT is applied over a large-spot, between 0.5 and 3.0 mm, and has a pulse duration of 1 minute (75-77). TTT uses an 810 nm near infrared laser in order to minimize damage to the nerve fiber layer (26). The technique has been shown to treat neoplasms, such as retinoblastomas, choroidal melanomas, and more recently, CSCR and choroidal neovascularization secondary to AMD (26-29). The goal of therapy is to maintain tissue hyperthermia without causing visible lesions, which is maintained by adjusting the treatment power to accommodate for differences in lesion size, pigmentation, macular elevation, and media clarity (26,76). Although the underlying pathophysiology behind TTT’s treatment effects is uncertain, one theorized mechanism are thermally induced changes in ocular blood flow (75). Several studies described benefits of TTT for CSCR eyes. Shukla et al. reported anatomical and functional improvement in eyes with chronic CSCR in up to 96% of cases with the use of 0.5 mm laser spot for 1 min and power ranged 60–120 mW in all cases (30). Wei et al. also applied TTT for the cases with longstanding leakage with favorable outcomes. In this technique a laser spot 1.2 mm diameter applied to the leak for a minute with a power of 120 mW. In contrast to conventional laser photocoagulation TTT as other subthreshold approaches can be applied for the cases with diffuse leakage (31). Dense subretinal hemorrhage, serous RPE detachment, and prior focal photocoagulation are a few identified contraindications for TTT. While adverse events are rare due to TTT’s subthreshold nature, they include decreased vision and retinal arteriole occlusion (76). While newer subthreshold techniques have shown promise in many retinal diseases, TTT’s efficacy in treating neoplastic tissues should not be ignored.
Traditional laser photocoagulation, anti-VEGF treatment, and photodynamic therapy (PDT) have been well-established standard interventions for many retinal diseases (78,79). Subthreshold laser has emerged as an alternate therapy and used in the clinical setting for central serious chorioretinopathy (CSCR), branch retinal vein occlusion (BRVO), AMD and DME (8,80-82). The technology for subthreshold lasers has been around since the 1970s, with the seminal report on this technology titled “Retinal Damage from Repeated Subthreshold Exposures Using a Ruby Laser Photocoagulator” (83). The study found that 0.5 millisecond laser pulses with an energy density of 375 micro-joules per square centimeter did not lead to independent damage, but rather had a cumulative effect. Several techniques have been developed since then to achieve the term “subthreshold”. However, the end goal of the technology is to ultimately produce a similar therapeutic effect of traditional photocoagulation without visible damage, or “burns” seen on ophthalmic exam. Subthreshold laser has also been explored for anterior segment diseases, particularly glaucoma such as micropulse laser trabeculoplasty (MLT) and pattern scanning laser trabeculoplasty (PSLT) (84,85).
CSCR is a common chorio-retinal disease characterized by subretinal fluid accumulation in the macular region causing patients to have blurred central vision. Its etiology remains incompletely understood but choroidal hyperpermeability appears to play an important role. Although acute CSCR is often self-limited and has a relatively good prognosis, chronic CSCR can lead to permanent vision loss (86). In extrafoveal leakage, traditional photocoagulation has shown to expedite the serous detachment, although treatment does not significantly improve visual outcomes or recurrence rate (87). Lanzetta et al. reported the use of subthreshold laser to treat 24 chronic CSCR eyes and found that subretinal fluid had resolved/improved in three quarters at the end of follow-up (ranging, 3–36 months). In addition, in most eyes there was no evidence of RPE changes due to the subthreshold laser treatment (7). Several studies compared the effectiveness of subthreshold laser to verteporfin PDT in chronic CSCR (88,89). These studies suggested that given the current lack of availability of verteporfin, subthreshold laser is a viable alternate mode of treatment for CSCR. CSCR is the first described entity of the pachychoroid disease spectrum, decreased macular thickness following SMPL treatment have been observed, however, SMPL does not influence the choroidal vasculature and thickness (90); furthermore, both PDT and SMPL do not significantly affect choroidal vessel index (91). A meta-analysis between SMPL and PDT for chronic CSCR was conducted and included four randomized clinical trials and five retrospective clinical studies found no significant difference in the effect of SMPL and PDT in CMT reduction, resolution of subretinal fluid, or adverse events (92). It is important to note that PDT may be a better therapeutic route for complex cases with longer duration and RPE alterations.
DME is a vision-threatening complication of diabetic retinopathy and a leading cause of blindness in the working age between 20–65 years old globally. The inner blood retinal barrier breakdown has been confirmed to be the primary pathogenesis of DME (93). Although anti-VEGF therapy is often the first line treatment for DME, laser photocoagulation has also been utilized to treat DME (94). According to the Early Treatment Diabetic Retinopathy Study Group, focal photocoagulation in DME has shown to decrease the risk of vision loss (95). Zas and colleagues (96), based on a review of the current literature, concluded that subthreshold laser therapy in DME was more effective in eyes with a central macular thickness ≤400 μm. Treatment should be delivered with a pattern of burns with no intervening spaces between burns. The mechanism of action appears to include downregulation of inflammatory biomarkers produced by Müller cells and activated microglial cells. Furthermore, subthreshold laser may decrease the anti-VEGF injection burden in selected patients. Combination therapy between SMPL and anti-VEGF injections is of high interest (97). A comparison study between SMPL + anti-VEGF injection combined therapy and anti-VEGF monotherapy for DME observed that combining anti-VEGF injections with SMPL led to a significant decrease in CMT at 6 months with less injections required (98). This study suggests that combination therapy may be a safe alternative while reducing the injection burden for DME patients. A randomized clinical trial with DME eyes that were resistant to anti-VEGF injection therapy reported that combining SMPL with anti-VEGF therapy can significant increase BCVA and reduce CMT for refractory DME (99). Subthreshold laser monotherapy in DME has shown to decrease intraretinal, fluid, subretinal fluid, and central macular thickness as well as an increase in BCVA. However, the improvements were considered mild, thus suggesting that combination therapy may be a more ideal approach in DME (11).
AMD is a common, progressive disease that leads to central vision loss. The etiology behind AMD is not completely understood but multiple risk factors have been identified including complement system dysfunction and VEGF upregulation, as well as genetic background (100). Traditional laser photocoagulation has been performed and studied in eyes with AMD, however, due to the disease location of AMD, photocoagulation and destruction of retinal tissue at the macula carries many risks as well as high recurrence rates (100). Thus, subthreshold laser has been studied as an effective intervention to attenuate the progression of AMD. The Laser Intervention in Early Stages of Age-Related Macular Degeneration Study Group conducted a study in intermediate AMD eyes with subthreshold laser and found that subthreshold laser may attenuate the progression of AMD in eyes without coexistent RPD (21). Luttrull et al. conducted a study with panmacular SMPL in high-risk dry AMD and observed a 47% slowed progression of age-related geographic atrophy (101). An additional study with SMPL in high-risk AMD found that SMPL therapy had a low incidence of choroidal neovascularization with no adverse effects (102). These encouraging outcomes support SMPL’s utility as an effective treatment for high-risk AMD. Further studies continue to investigate the effectiveness of subthreshold laser in AMD patients, including the reduction of drusen and vision improvement (73,103,104).
BRVO often leads to macular edema which can lead to vision loss (105). Primary treatment for macular edema due to RVO include intravitreal corticosteroid and anti-VEGF therapy (106). Laser photocoagulation has historically been utilized to treat persistent macular edema due to BRVO, but recent studies have shown that anti-VEGF agents are more effective in improving BCVA, reducing central retinal thickness, and lowering the risk of elevating intraocular pressure (107). Due to its safer profile, subthreshold laser has been studied as a possible alternative and/or combination therapy (108). SML has been shown to be safer and as effective as intravitreal injections of ranibizumab for ME-BRVO (108). Inagaki et al. conducted a study with subthreshold laser in persistent macular edema secondary to BRVO and found that subthreshold laser was effective in controlling the macular edema (109). In a randomized controlled trial, Parodi et al. found that subthreshold laser treatment combined with intravitreal triamcinolone injection resulted in significant visual acuity improvement in macular edema from BRVO (110). Terashima et al. reported that combining subthreshold laser and intravitreal ranibizumab (anti-VEGF) was effective in maintaining visual acuity in macular edema secondary to BRVO while decreasing the frequency of injections (111). These studies are promising for subthreshold laser as a combination therapy for macular edema due to RVO and encourage further studies for this therapy.
Subthreshold laser systems have undergone significant advances in the past three decades, as multiple companies have competed to create optimized laser products that improve patient outcomes. In addition to the development of novel subthreshold modalities, the core parameters have changed, as different lasers and wavelengths are being used today compared to a few decades ago (8). Companies have also focused on including a plethora of add-ons and assistive technologies to improve accessibility and ease of use. Recently, commercial devices boasting multi-functionality have become commonplace, with certain devices able to perform YAG capsulotomy, selective laser trabeculoplasty, and SMPL all from one device (112). The focus of this section will be on the retina applications of these subthreshold laser systems, although many of the systems are able to treat anterior segment diseases. Table 3 compares the specifications of several commercially available subthreshold lasers (Table 3).
| Parameter | Device name | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclo G6 | IQ 532 | IQ 577 | OcuLight SLx | PASCAL Synthesis | Navilas Prime | Navilas Pro | EasyRet | Vitra 810 | Digital Trio | Selecta Trio | Smart 532 | R:GEN | |
| Currently sold | Y | Y | Y | Y | Y | Y | Y | Y | Not in the USA | Y | Y | Y | Y |
| Weight (kg) | 4.8 | 9.0 | 9.0 | 6.3 | 15.0 | – | – | 60.0 | 5.6 | <64 | <64 | 4 | – |
| Laser type | Solid-state | Solid-state | Solid-state | Solid-state | Optically pumped semiconductor | Optically pumped semiconductor | Optically pumped semiconductor | Fiber laser | Solid-state | Q-switched Nd:YAG, frequency doubled for selective laser trabeculoplasty | Q-switched Nd:YAG, frequency doubled for selective laser trabeculoplasty | Diode-pumped solid state | Q-switched Nd:YLF |
| Retina | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Glaucoma | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | N |
| Capsulotomy | N | N | N | N | N | N | N | N | N | Y | Y | N | N |
| Digital display | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Touch screen interface | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Pattern lasering | N | N | N | N | Y | Y | Y | Y | Y | N | N | Y | N |
| Recording capabilities | N | N | N | N | N | Y | N | Y | N | Y | N | N | N |
| Retina | |||||||||||||
| Subthreshold? | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Technology | – | Subthreshold micropulse laser | Subthreshold micropulse laser | Subthreshold micropulse laser | Endpoint management | Subthreshold micropulse laser | Subthreshold micropulse laser | Subthreshold micropulse laser | – | Subthreshold micropulse laser | Subthreshold micropulse laser | Subthreshold micropulse laser | Selective retina therapy |
| Pulse length (ms) | – | 0.05–1 | 0.05–1.0 | 0.1–1.0 | NA | 0.05–0.5 | 0.05–0.5 | 10 ms to continuous | – | 0.05–0.5 | 0.05–0.5 | 0.05–0.5 | 1.7 μs |
| Pulse interval (ms) | – | 1.0–10.0 | 1.0–10.0 | 1.0–10.0 | NA | – | – | – | – | 0.3–10 | 0.3–10 | 0.3–10 | – |
| Duty cycle | – | 5%, 10%, 15% | 5%, 10%, 15% | 0.4–50% | NA | 5%, 10%, 15% | 5%, 10%, 15% | 5–100% | – | 5%, 10%, 15% | 5%, 10%, 15% | 5%, 10%, 15% | 0.00017 |
| Power (mW) | – | 0–2,000 | 0–2,000 | 0–3,000 | 0–2,000 | 50–2,000 | 50–2,000 | 0–2,000 | – | 0–2,000 | 0–2,000 | 0–2,000 | – |
| Wavelength (nm) | – | 532 | 577 | 810 | 532 or 577 | 577 | 577 | 577 | – | 532 | 532 | 532 | 527 |
| Glaucoma | |||||||||||||
| Subthreshold? | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | N | N |
| Technology | Micropulse transscleral cyclophotocoagulation | Micropulse laser trabeculoplasty | Micropulse laser trabeculoplasty | Micropulse laser trabeculoplasty | Micropulse laser trabeculoplasty | Micropulse laser trabeculoplasty & pattern scanning laser trabeculoplasty | Micropulse laser trabeculoplasty & pattern scanning laser trabeculoplasty | – | Micropulse laser trabeculoplasty | – | – | – | – |
| Pulse length (ms) | 0.05–1.0 | 0.05–1 | 0.05–1.0 | 0.1–1.0 | NA | NA | NA | – | 10 ms to continuous | – | – | – | – |
| Pulse interval (ms) | 1.0–10.0 | 1.0–10.0 | 1.0–10.0 | 1.0–10.0 | NA | NA | NA | – | – | – | – | – | – |
| Duty cycle | 0.5–50% | 5%, 10%, 15% | 5%, 10%, 15% | 0.4–50% | NA | NA | NA | – | 5–35% | – | – | – | – |
| Power (mW) | 50–3,000 | 400–1,200 | 400–1,200 | 0–3,000 | 0–2,000 mW, 3.4 mJ | 50–2,000 | 50–2,000 | – | 0–3,000 | – | – | – | – |
| Wavelength (nm) | 810 | 532 | 577 | 810 | 532 or 577 nm | 577 | 577 | – | 810 | – | – | – | – |
N, no; Y, yes.
IRIDEX is a company that specializes in developing laser-based medical systems (122). The company’s OcuLight SLx System using an 810 nm laser with their MicroPulse technology was the first subthreshold laser to be used clinically. In a nonrandomized clinical study involving 53 patients with choroidal neovascularization, 14 patients with macular edema from BRVO, and 59 patients with DME, the MicroPulse 810-nm laser was effective in the treatment of all three groups (123). This breakthrough in subthreshold technology led to a plethora of new systems and companies developing their own SMPL systems. However, despite its initial success, the laser failed to improve BCVA or reduce choroidal neovascularization for prophylactic treatment of AMD during a multicenter, randomized, clinical trial (124,125). Since this study, the company has researched ways to improve and optimize their design.
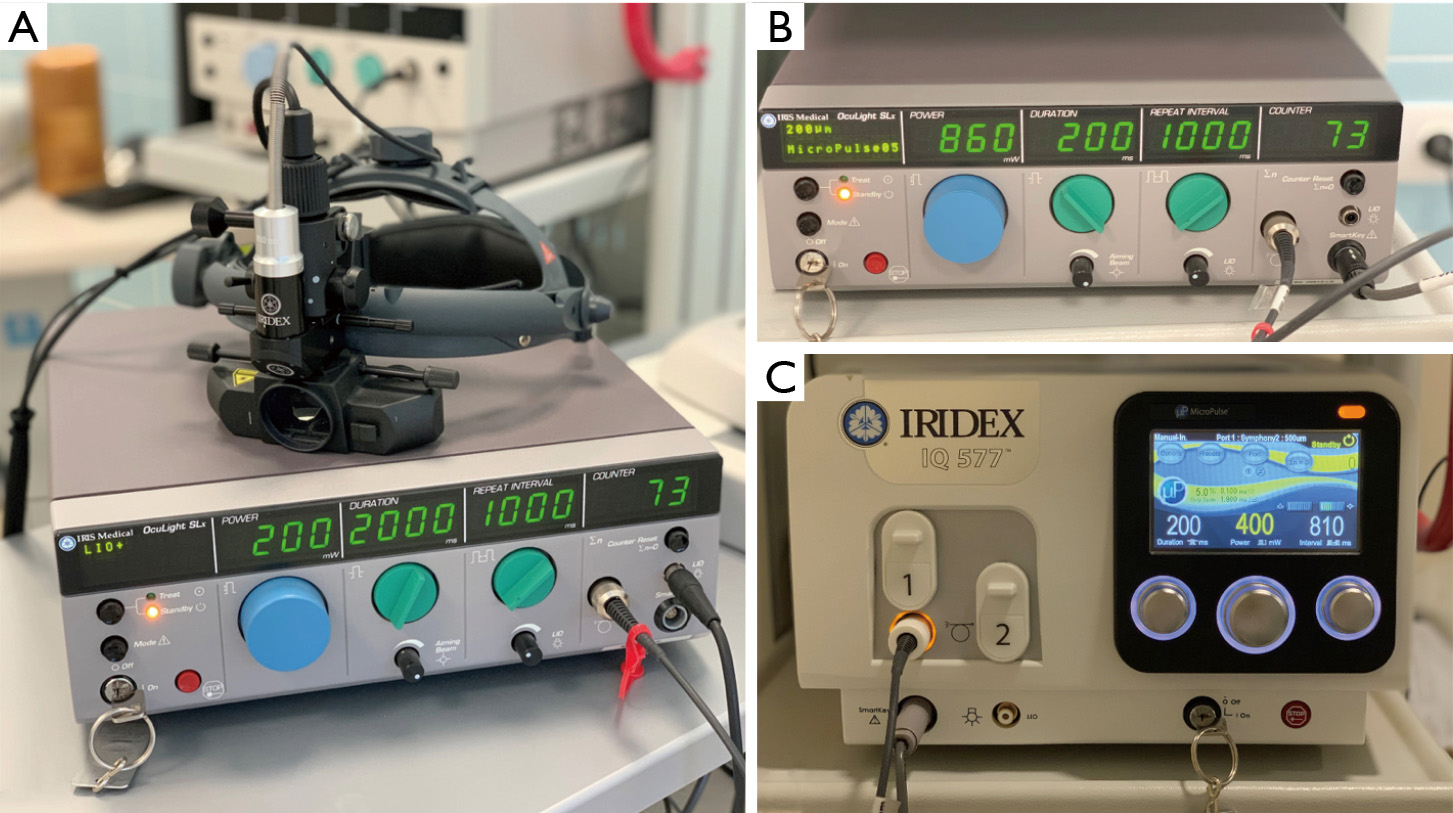
While IRIDEX has primarily created lasers for retinal treatment, the company has also introduced their micropulse technology for glaucoma applications (113). Recently, IRIDEX has also expanded its scope through the acquirement of Topcon’s PASCAL laser technology (114). The PASCAL laser system offers EpM which controls the power and duration of the continuous laser to allow for subthreshold effects. The system can also perform PSLT which allows for subthreshold applications in glaucoma procedures (115). Their systems include the Cyclo G6, IQ 532, IQ 577, OcuLight SLx, and PASCAL Synthesis (Table 3, Figure 3).
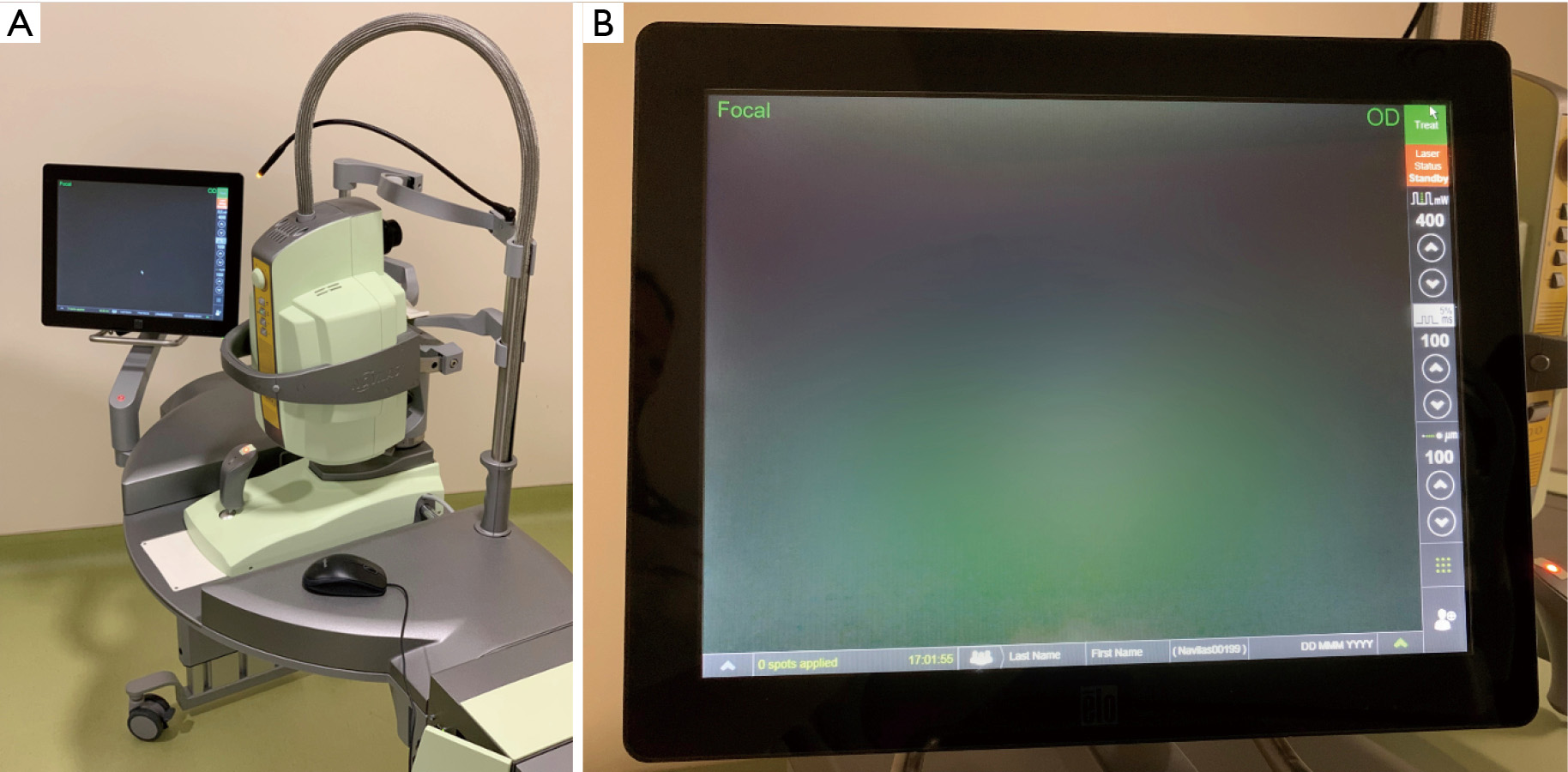
OD-OS is a company that primarily specializes in retina treatment (116). The Navilas Laser System utilizes the OD-OS Retina Navigation platform and integrates imaging, planning, treatment, and documentation. The company has also integrated subthreshold therapies into their laser system. The Retina Navigation platform allows for systematic integration of real-time imaging, digital planning, treatment overlay, and documentation (17). The live fundus imaging allows for proper planning as desired treatment locations can be marked on the image. Additionally, the device displays caution zones to allow for better safety during treatment. After the procedure is complete, a digital report is created for future reference (117). Over the last decade, OD-OS has released numerous products from the Navilas product line including the Navilas System, Navilas 532, Navilas 577, Navilas Navigated Microsecond (Figure 4).
Quantel Medical is an ophthalmic device company primarily focuses on developing lasers in ophthalmology (118). Quantel Medical introduced its first solid-state photocoagulator, Viridis, in 1995, which started to bring the era of argon laser in ophthalmology to an end. It then released the Activis and the Iridis for AMD treatment and YAG Optimis for capsulotomy in 1998. In 2009, the company introduced the world’s first 577 nm multiplot pattern scanning laser with a subthreshold mode, the Supra Scan 577. In 2016, Quantel Medical released the EasyRet which is their retinal laser, a 577-nm yellow photocoagulator that utilizes fiber laser technology with subliminal emission mode (118). Quantel Medical also has the Vitra 810 which is used for end-stage glaucoma.
Lumenis, originally Coherent is a company with a 50-year history of producing lasers. Coherent created the first medical laser for ophthalmology and was first to commercialize a laser-based system (119). In 2002, the company introduced Selecta Duet, which was the first combination laser for SLT and YAG. In 2016, the company merged with XIO Group and released the Smart 532 Laser with subthreshold capabilities (119). Lumenis offers subthreshold lasers via SMPL. These modalities are currently offered through the Digital Trio and Duet series, the Selecta Trio and Duet series, and as the standalone Smart 532 Laser.
Lutronic Vision is a company that specializes in SRT lasers. The company has been building prototypes and researching SRT lasers since 2010 and has been executing clinical trials since 2014. Their laser system, R:GEN, has been FDA approved for clinically significant macular edema in 2016 and is currently undergoing clinical trials for other retinal diseases (120). R:GEN is currently the only SRT device approved for clinical use. The device uses a 527-nm laser with a 1.7 microsecond pulse duration (121). The device also contains real-time feedback through their Dual Dosimetry Technology to monitor RPE cell destruction and achieve a better safety profile. The company’s Auto Ramp-up Technology allows for incremental pulse energy ramping for precise control and termination of treatment (121).
Subthreshold laser has an encouraging future in effectively treating retinal diseases. In addition to current systems for clinical use, there are also multiple systems in development. In this final section we detail some of the upcoming subthreshold laser technology for treating retinal diseases in the future (Table 4). As recommended by many authors of subthreshold laser studies, it is encouraged to continue conducting prospective research to further optimize subthreshold laser for retinal diseases.
| Parameter | Device name | ||
|---|---|---|---|
| 2RT | Unnamed prototype | SPECTRALIS Centaurus | |
| Currently sold | N | N | N |
| Weight | – | – | – |
| Laser type | Q-switched Nd:YAG | Q-switched Nd:YLF | – |
| Retina | Y | Y | Y |
| Glaucoma | N | N | N |
| Capsulotomy | N | N | N |
| Digital display | Y | – | N |
| Touch screen interface | Y | – | N |
| Pattern lasering | N | N | N |
| Recording capabilities | N | N | N |
| Retina | |||
| Subthreshold? | Y | Y | Y |
| Technology | Subthreshold nanosecond laser | Selective retina therapy | Selective retina therapy |
| Pulse length | 3 ns | 1.7 μs | – |
| Pulse interval | – | – | – |
| Duty cycle | – | 0.00017 | – |
| Power | – | – | – |
| Wavelength (nm) | 532 | 527 | 532 |
N, no; Y, yes.
AlphaRET was established in 2020 and focuses on the Retinal Rejuvenation Therapy 2RT laser which uses SNL (126). However, this device is still undergoing the FDA approval process in the USA (127).
Medical Laser Center Lübeck is a research and development company that has been developing SRT prototypes and running clinical studies (128). The company has conducted various clinical studies to validate SRT, with their most recent study reporting favorable visual outcomes in CSCR without risk of scotoma (129).
Heidelberg Engineering is a company focused on imaging and software technologies for ophthalmic applications (122). They have partnered with the optoLab of the Bern University of Applied Sciences in Biel to develop the SPECTRALIS Centaurus system. This device integrates an OCT real-time imaging system with a retinal treatment laser to provide SRT (130). Additionally, the software can be extended to automate treatment via mapping of a predefined region. This device is a prototype and is still undergoing experimentation (130).
Subthreshold lasers have emerged as useful and powerful therapy for managing various retinal diseases. Current research ranging from biomarker research to clinical studies continue to shed light on the mechanisms and therapeutic benefits of this technology. Subthreshold laser systems are being further optimized to treat retinal diseases, such as caution zones and real-time feedback, as well as treating various additional ocular pathologies including glaucoma. Companies are also developing prototypes in subthreshold lasers, moving towards a future with more available systems for retinal diseases. Further research on these developing and established systems will provide further insight into the mechanisms of this technology. These advances highlight the therapeutic utility of subthreshold laser systems and its role in managing retinal diseases.