Abstract: Animal models are crucial for the study of tumorigenesis and therapies in oncology research. Though rare, uveal melanoma (UM) is the most common intraocular tumor and remains one of the most lethal cancers. Given the limitations of studying human UM cells in vitro, animal models have emerged as excellent platforms to investigate disease onset, progression, and metastasis. Since Greene’s initial studies on hamster UM, researchers have dramatically improved the array of animal models. Animals with spontaneous tumors have largely been replaced by engrafted and genetically engineered models. Inoculation techniques continue to be refined and expanded. Newer methods for directed mutagenesis have formed transgenic models to reliably study primary tumorigenesis. Human UM cell lines have been used to generate rapidly growing xenografts. Most recently, patient-derived xenografts have emerged as models that closely mimic the behavior of human UM. Separate animal models to study metastatic UM have also been established. Despite the advancements, the prognosis has only recently improved for UM patients, especially in patients with metastases. There is a need to identify and evaluate new preclinical models. To accomplish this goal, it is important to understand the origin, methods, advantages, and disadvantages of current animal models. In this review, the authors present current and historic animal models for the experimental study of UM. The strengths and shortcomings of each model are discussed and potential future directions are explored.
Uveal melanoma (UM) is the most common intraocular malignancy in adults, accounting for 3–5% of all melanomas (1). Over 90% of tumors are located in the choroid (2). Even though several diagnostic and therapeutic modalities for UM exist, many suffer from metastases and frequently succumb to the disease. Human UM has been difficult to study for several reasons. It is considered a rare cancer, affecting only 5 individuals per million per year worldwide (3). Given the frequent use of plaque brachytherapy in treating UM, the enucleation rate has decreased, limiting available eyes for investigation. Furthermore, human UM cell lines are limited in quantity and are expensive. Thus, animal models have been used for several decades to study UM growth and dissemination. Many models have been shown to recapitulate human UM, presenting platforms to study both tumorigenesis and treatment.
The ideal animal model for UM should reflect the pathogenesis and cellular behavior of human UM. There should be similarities in structure and behavior between the animal eye and human eye. The pathogenesis and evolution of UM should be as similar as possible to human UM. Additionally, tumor production in vivo should be as high as possible to maximize tissue yield and minimize number of animals. Finally, the size of the animal eye should be large enough to permit photographic study and follow-up of the outcomes of interest (4). These are crucial to allow extrapolation of the results from the model to humans.
Several animal models exist to study primary and metastatic UM. Those for primary UM can be divided into spontaneous, engrafted, and transgenic models. Spontaneously occurring UMs have been reported in many other animals. Xenograft and transgenic models provide an excellent avenue to explore growth of primary UM (5-8). Engrafted models can be made via inoculation of malignant cells in the healthy animal. Rapid tumor production in these animals allows for the study of disease using non-invasive imaging (9). Finally, genetically engineered models provide information about pathways that affect tumor initiation and metastasis. Viral vectors, teratogens, and specific promoters for ocular tissues are some methods used for these models. By studying directed tumorigenesis in healthy animals, they can elucidate the contribution of specific mutations on disease progression (10).
In this article, we present the most common animal models in primary UM and briefly discuss models for metastatic UM. We highlight the basic techniques required to generate them and focus on the advantages and disadvantages of each model.
Spontaneous models represent the animals which develop UM under natural conditions. UM in dogs was first described in 1919 as a case of melanotic sarcoma in the dog choroid (11). Over the years, investigators have reported the occurrence of UM in rats, cattle, cats, pigs, fish, and chicken but the dog has been the most well-studied (12).
Ocular melanoma represents the most common primary intraocular tumor in dogs (Figure 1). Studies examining over 60 dog breeds have shown canine and human UM share many common features like clinical presentation, but canine UM most commonly arises in the anterior uvea (12-14). Distant metastasis is also rarely documented (12,14). Cats have also been investigated, with one group noting buphthalmia, hyperpigmentation of iris and ciliary body, and ophthalmitis in 16 cats with UM. Enucleation and subsequent sacrifice of these cats showed that 63% developed metastases to the brain, lungs, and liver, although the tumors did not harbor canonical human UM mutations (15). Spontaneous occurrence of ocular melanoma in rats and rabbits are rare, even though these animals are commonly used in UM research.
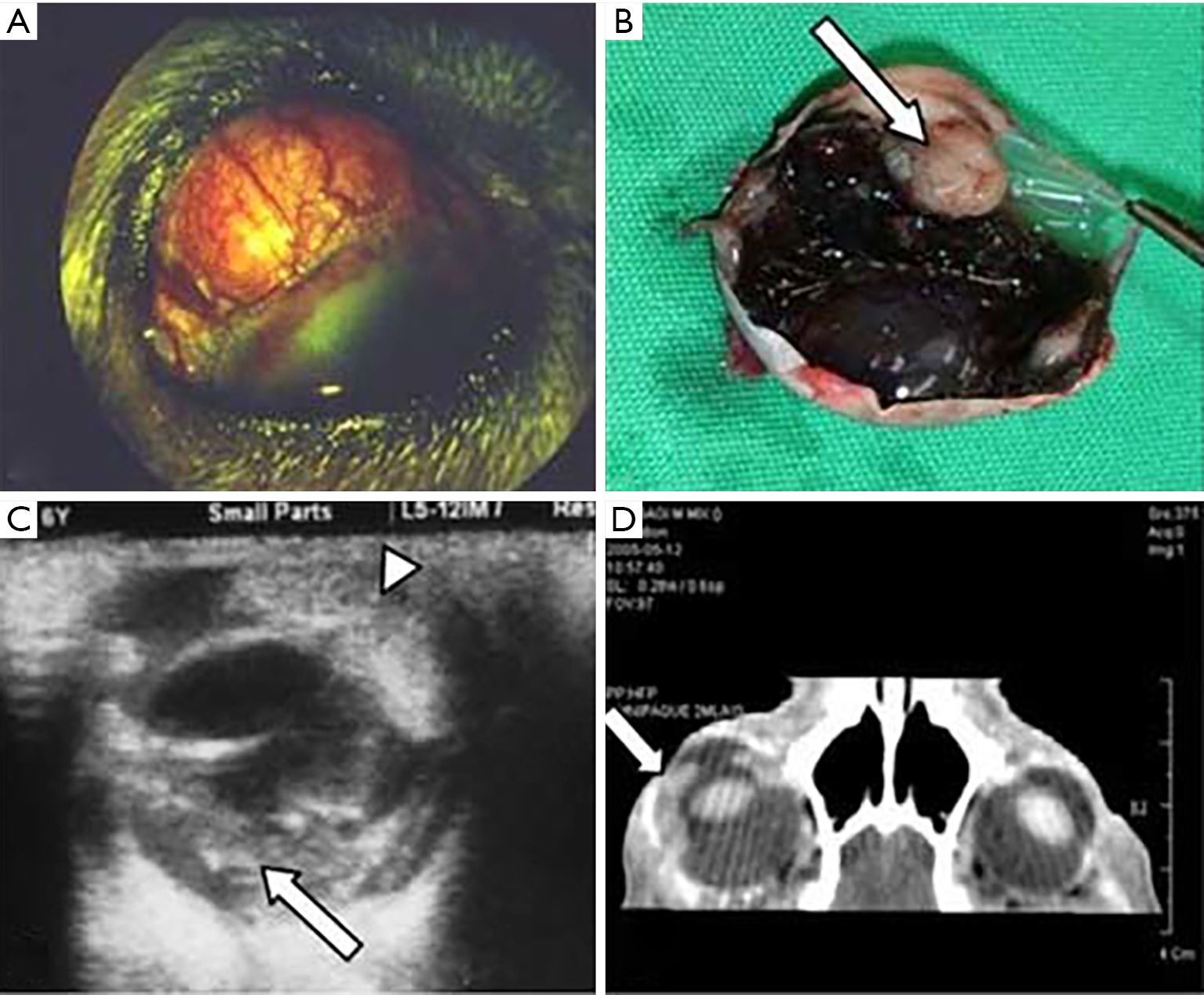
Spontaneous UM are difficult to study, as the tumors have an unpredictable incidence rate and follow an inconsistent metastatic pattern. Limited numbers of such animals further restrict their use. The greatest benefit of using such spontaneous models is the ability to manipulate the natural occurrence of UM in the presence of an active immune system.
An engrafted model can be formed by inoculating melanoma cells in the model eye. Animal cutaneous melanoma cells placed into the model eye are defined as “heterotopic” transplantations. In contrast, human UM cells placed into the eyes of animals are defined as “orthotopic” models. In this section, we present delivery methods, cell lines, and animals used to emulate human UM.
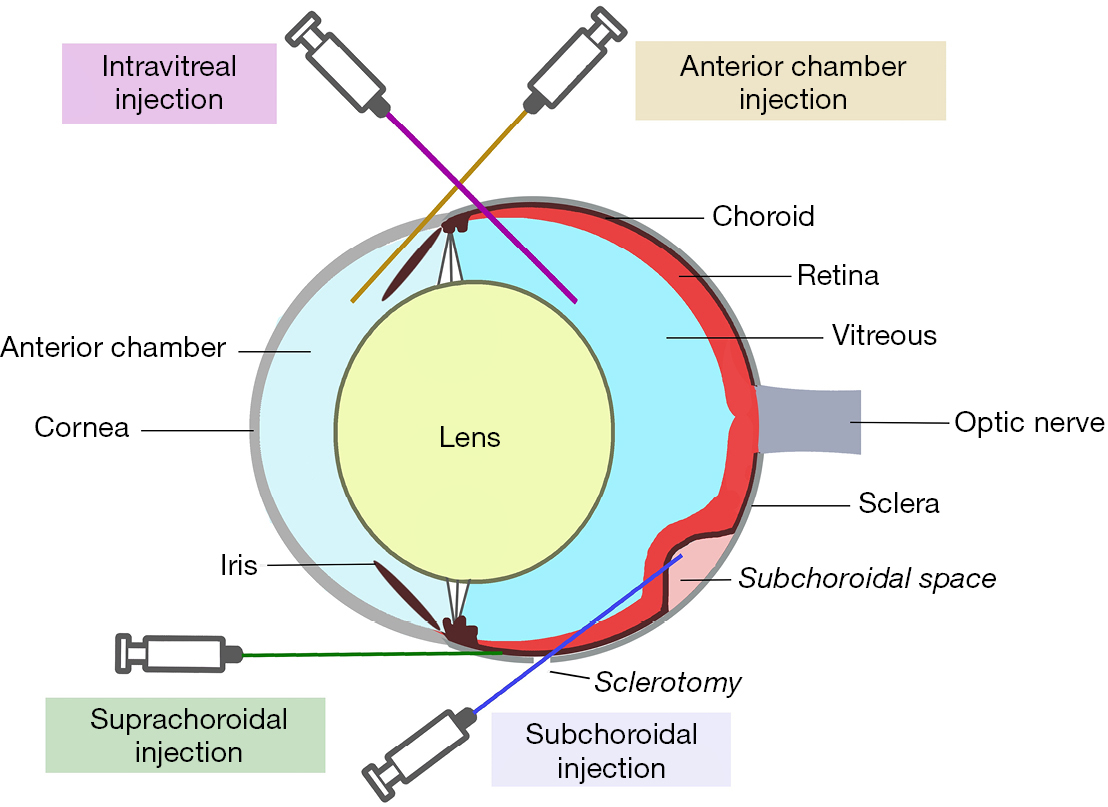
Inoculation can be conducted inside a compartment of the eye or in an extraocular tissue, such as subcutaneous or in a fat pad. The latter method can be feasible for studying tumor growth and local therapy response, but it may not accurately simulate the ocular environment. Inoculation of cells into the eye allows the study of UM cells that do not grow well outside the eye. Cells are usually inoculated into different compartments: the anterior chamber (AC), the suprachoroidal space, the subchoroidal space, and the vitreous (Figure 2). All methods of inoculation are frequently followed with enucleation after 5–10 days for histopathological analysis and animals are sacrificed 3–4 weeks after inoculation to examine for metastases. A comparison of different methods is outlined in Table 1.
| Site | Description | Strengths | Limitations | References |
|---|---|---|---|---|
| AC | Implantation of tumor cells through a corneal defect into the AC | Reliable formation of iris melanomas in an immune-privileged space |
Metastases limited and cells can seed extraocular space | In hamsters (16-20) |
| In mice (21-32) | ||||
| In rabbits (33,34-39) | ||||
| Suprachoroid | Transcorneal or transconjunctival delivery of cells directly above the choroid | Reliable formation of choroidal melanoma with metastases. Transcorneal approach has minimal extraocular tumor growth. | Deep corneal stromal perforation in transcorneal approach and seeding of cells into the subconjunctival space in transconjunctival approach. | In mice (23,29,40-51) |
| In rabbits (52-60) | ||||
| In rats (61,62) | ||||
| Subchoroid | Placement of cells inside an iatrogenic choroidal detachment after retinotomy | Reliable formation of choroidal melanomas within a short period of time | Complex technique with retinotomy. Complications such as vitreous hemorrhage, vitreous cell seeding, retinal detachment, and proliferative vitreoretinopathy. | In hamsters (63) |
| In rabbits (37,64-69) | ||||
| Intravitreal | Injection of tumor cells directly into the vitreous through the sclera | Effective primary tumor growth in the choroid | No extrapulmonary metastases. Changes in the tumor environment. Possible seeding into the anterior chamber. | In mice (70-73) |
AC, anterior chamber.
Historically, the AC of the eye has been the preferred site of tumor implantation due to its immune privilege (74). A method of AC tumor implantation was demonstrated by Niederkorn and colleagues (75). A 30-gauge needle is used to puncture the cornea at the corneoscleral junction anterior to the iris. This is key as iris prolapse and subsequent plugging of the perforated cornea reduces fluid leakage when the needle is withdrawn. After aqueous humor expression, an 80-μm glass micropipette is fitted into an infant feeding tube (No. 5 French; Cutter Laboratories, Inc., Berkeley, CA) and mounted on a Hamilton syringe (Hamilton Co., Inc., Whittier, CA). A Hamilton automatic dispenser is fitted onto the syringe and the pipet is loaded with a cell suspension and introduced into the AC through the corneal defect. This method has been successful especially in murine models (21-23).
Suprachoroidal administration via transcorneal and transconjunctival approaches into the posterior compartment (PC) of eyes have been described (76). The PC region consists of the choroid, ciliary body, and subretinal space and should not be confused with the posterior chamber. The transcorneal technique starts with a tunnel from the limbus within the cornea, sclera, and ciliary body to the choroid using a 30-gauge needle. Cells are loaded similar to that in the AC approach in a 10-μL glass syringe. The tip of the syringe with a 33-gauge Hamilton metal needle (Hamilton Co., Inc., Whittier, CA) is inserted and the cells injected. The iris closes the perforation when the needle is removed. Care must be taken to limit the incision to the cornea, sclera, and ciliary body, as the deep corneal stroma can perforate and contaminate the AC with tumor cells (76). The transconjunctival technique is employed using a 30-gauge needle inserted 1 mm posterior to the limbus through the conjunctiva and sclera. Cells are prepared and inserted like the transcorneal approach. Though this technique is successful, a disadvantage is that the cells can escape into the subconjunctival space and result in primary extraocular melanoma (76).
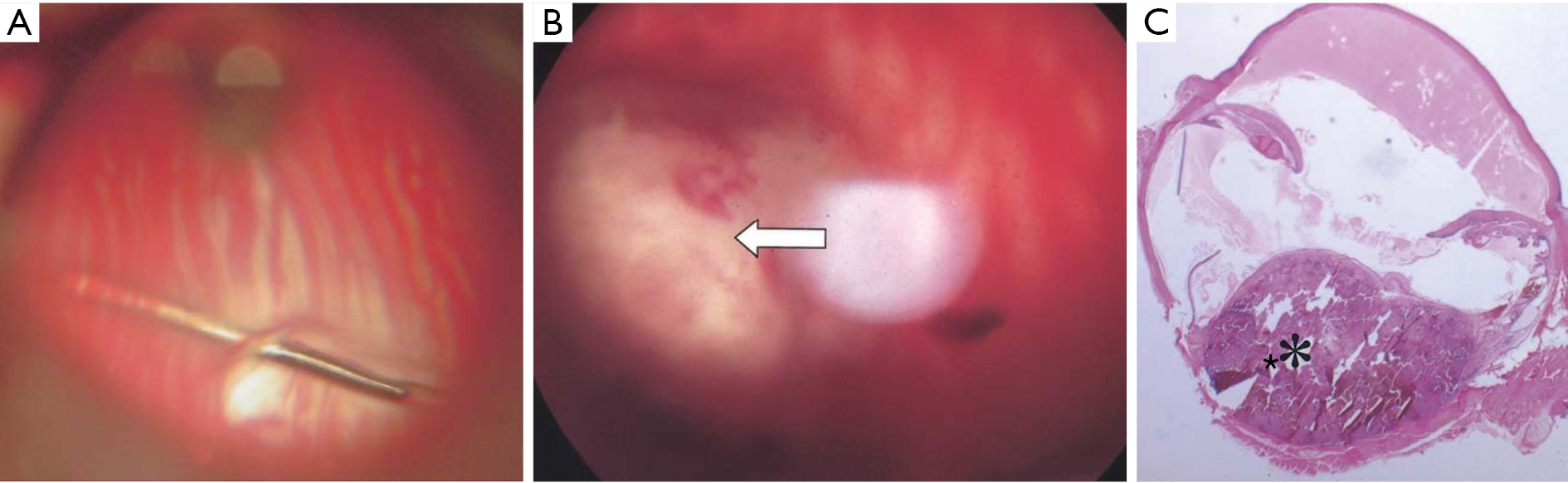
More recent methods involve introducing tumor cells into the vitreous or subchoroidal space. The mice and cell suspensions are prepared similarly as in the suprachoroidal approach. Kilian et al injected HCmel12 melanoma cells 1 mm posterior to the limbus through the sclera into the vitreous. Mice were enucleated at day 9 and euthanized at day 42. Metastatic lesions in the lungs, liver, and spleens were seen in some of mice (70). First described in rabbits in 2004, the subchoroidal technique involves the creation of a sclerotomy 1 mm posterior to the limbus and performing a small retinotomy using a 33-gauge cannula. Subsequently, viscoelastic is injected with the same cannula into the subchoroidal space, producing a choroidal detachment (Figure 3). The cell suspension is placed inside the space opposite to the retinotomy to prevent vitreous seeding and the sclerotomy is closed. This technique has successfully formed choroidal melanomas approximately 5 optic disc diameters in size after 1 month (33).
Several cell lines have been established to study UM. Table 2 provides an overview of the cell lines used in different animal models. The advantages and disadvantages of each host-cell line pair should guide investigators in choosing in the model that will be most suitable in answering research questions related to tumor genetics, histopathology, biology, metastatic patterns, or imaging features.
| Model | Cell line | Immunosuppression | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Hamster | Greene | Not required | Histologic similarity to human UM; naturally occurring | Cutaneous in origin; hemorrhagic necrosis; poor suitability to study metastases | (16,17,63,77,78) |
| Bomirski | Not required | Spontaneously occurring; reliable formation of metastases | Cutaneous in origin; no hepatic metastases; limited availability | (18-20) | |
| Murine | B16-LS9 | Not required | Widespread availability; histologic similarity to human UM; diffuse metastases to the liver and other organs | Cutaneous in origin | (28,41-51,71,72,79-81) |
| B16-F10 | Not required | Reliable primary tumor formation; rapidly growing; NK-sensitive; imaging conducted in literature | Variable visceral metastases with AC inoculation; no metastases with suprachoroidal | (21-24,27-30,76) | |
| B16-F10 Queens | Not required | Higher tumor formation rate than B16F10; pulmonary metastases | Variable extra-pulmonary metastases | (23,25-27,40) | |
| HCmel12 | Not required | VM pattern of human UM; pulmonary and regional lymph node metastases | Cutaneous in origin; literature limited | (70,73,82) | |
| Human | Required | Tumors similar in genetic background; reliable production of hepatic metastases; high tumor engraftment rate | Validation of origin; genetic instability in vitro; high cost | (9,31,32,83-91) | |
| Rabbit | Greene | Not required | Rapid tumor growth | No metastases with AC inoculation; hemorrhagic necrosis; poor suitability to study metastases | (33-35,52,64,65,92) |
| B16-F10 | Required | Reliable primary tumor formation; rapidly growing; pulmonary metastases | Variable extra-pulmonary metastases | (36,37,66-68,93) | |
| Human | Required | Highly aggressive choroidal melanoma formation; pulmonary metastases in PC approaches | No metastases with AC inoculation; variable hepatic metastases with PC techniques | (38,39,53-60,69) | |
| Rat | Human | Required, but nude athymic rat used | Tumor behavior like human UM, athymic rat has no immune system; high tumor formation with spheroids; imaging feasible | Not widely used; no report of metastasis formation | (61,62) |
UM, uveal melanoma; AC, anterior chamber; PC, posterior compartment; VM, vasculogenic mimicry.
One of the most established UM cell lines is the Greene melanoma, which forms amelanotic cutaneous melanomas in hamsters (94). A significant advantage of this cell line is that light and electron microscopic features of the melanoma are similar to those of human UM (95). The immunohistochemical reaction pattern is comparable and has been widely used to assess factors for tumor growth and response to treatment (76,96). Furthermore, it is one of the cell lines that does not need immunosuppressive treatment for growth. Unfortunately, the cell line has a propensity for hemorrhagic necrosis, seen at 8 to 10 days after AC inoculation. Immunosuppressive treatments can be used to slow necrosis, but significant inflammation is still reported to occur (76). This is also not a tumor of ocular origin, which limits generalizability of findings to human UM.
The B16 melanoma is a C57BL mouse cutaneous melanotic melanoma line. Unlike Greene melanoma, this cell line has pigmented cell strains and has been predominantly used to study UM metastasis (23). The original cell line was named B16 line F1 (first passage) by Fidler, but several subcultures have been created via propagation or directed manipulation (97). The B16-F10 line is the 10th propagated line (21,22,24). The B16-F10 Queens is a more aggressive cell line (23,25-27,40). Finally, the B16-LS9 is enriched for hepatic metastases (41-50,71,72,79,98). These cells have high metastatic potential, reliable production of visceral metastases, and are well-characterized. Unlike the Greene melanoma, this model produces metastasis at a predictable rate and allows for the study of tumor immunology (83). However, these cells are not of uveal origin.
Several cell lines from both primary and metastatic UM have been established over the past decade (42,99-107). The origins of some of these cell lines have been studied extensively (84). The primary advantage of these cells is that the cells are directly inoculated from humans and have the potential to study human UM behavior more accurately than any animal cell line. As they often have key driver mutations, these models closely recapitulate the behavior, development, and mutagenesis of human UM. Shortcomings of these cell lines are the cost and inherent genomic instability.
Non-human cutaneous melanoma cells have been greatly utilized in earlier studies of UM. The generalizability of data has been markedly limited by its cutaneous origin and variable metastatic features. However, researchers continue to obtain valuable information from identified similarities in genetics, pathology, and biology of animal and human melanomas.
Hamster eyes have been used to study UM for decades. Their eyes are small, which limits routine eye assessment and surgery. Despite this shortcoming, there are several established models in the literature to study tumorigenesis and treatment response.
An ocular melanoma model was described in 1961 using Greene melanoma cells. Researchers inoculated 2 to 4 μL of cells into the AC of Syrian gold hamsters using the method described above, creating tumors that grew into the orbit and resulted in lymph node and visceral metastases (16). Another group inoculated pieces of melanoma cells onto the surface of the hamster iris, producing rapidly growing iris melanomas (17). In addition to AC administration, subchoroidal inoculation of Greene cells have been reported. Researchers demonstrated that injection of 5 μL of 200 cells/mL of Greene melanoma cells into the choroid reliably produces melanoma. In that study, the hamster eyes had histological evidence of choroidal melanomas 2 weeks after injection, though the majority showed evidence of necrosis and scleral perforation. Visceral metastases in multiple organs were evident 6 weeks after injection (63).
Researchers have also used this cell line to study treatment response. A group from the Netherlands conducted one of the first studies for transpupillary thermotherapy (TTT) using Greene melanoma cells on hamsters. They performed subcutaneous inoculation of Greene cells on the Syrian golden hamster and irradiated the tumors at sub-photocoagulation level of 45–60 ℃, showing necrosis even after 1 minute of therapy (77,78). These promising results encouraged them to start exploring TTT for human UM.
There are distinct benefits of using the Greene melanoma cell line in the hamster: it is a naturally occurring melanoma and rapidly grows within many regions of the hamster eye. The tumor also does not require immunosuppressive treatment and demonstrates histologic components analogous to human UM (76). The drawback of this particular cell line in the hamster is its limited availability.
In 1988, Bomirski and colleagues reported a family of spontaneous cutaneous melanomas in the Syrian golden hamsters (18). Investigators inserted cells from these tumors into the AC of hamsters that resulted in rapidly growing and metastasizing melanomas. Pigmented metastases were found in the lungs and amelanotic metastases were found in the kidneys (19). Another group performed AC inoculation and found the melanomas to have high vascularity and ability for both regional and visceral metastases (20). The drawback for this cell line is its limited use and availability worldwide.
The laboratory mouse is a strong animal model to study UM. It is widely available, inexpensive, and has a rapid reproduction rate. Similar to hamster eyes, mice eyes are small and may pose difficulties in ophthalmic examination and surgery. Another advantage is that the genomic profile of mice shows great similarity with humans, which increases the likelihood of its behavior and applicability to human UM.
Perhaps the most widely used mouse model of UM is created via inoculation of the B16-LS9 melanoma cells into the eyes of C57BL/6 mice. This subculture of B16 cells was developed by Rusciano and colleagues, who collected the cells after repetitive injections of intrasplenic UM cells (79). AC, suprachoroidal, and intravitreal injections of these cells have been described. AC inoculation can successfully form iris melanomas, which are less likely to form metastases compared to methods that deliver the inoculum to the PC (28). The suprachoroidal approach can form choroidal melanomas and have been successful in several studies investigating pathogenesis of UM and tumor immunology, reporting widespread metastases to the liver, lungs, and regional lymph nodes (41-49,51). The transcorneal technique was also illustrated in this model, where the authors reported extraocular melanomas in 43% of eyes, compared to 100% in eyes for the transconjunctival technique (50). Intravitreal injections have also been used to inoculate these cells and form choroidal melanomas metastatic to the liver (71,72). Tumor growth can also be successfully followed using non-invasive imaging techniques such as ultrasound and optical coherence tomography (80).
The advantages of using this cell line in mice include reliable tumor and metastasis formation, widespread availability, and established presence in literature. The findings from the metastases generated from this model can have applicability in humans, as the histological growth pattern of metastases are similar to those with metastatic human UM (81). The primary drawback is that the tumor is cutaneous in origin. Murine UM cell lines that can be acquired from genetically engineered mice may overcome this limitation.
The B16-F10 cell line has been inoculated into C57BL/6 mice. Suprachoroidal and AC approaches have been used to successfully grow intraocular tumors. Mice sacrificed 4 weeks after AC inoculation have shown a pulmonary metastasis rate of 0 to 33% (23,28,29). The AC method has been used more frequently but has shown mixed results in metastases formation. As the B16-F10 cells is thought to be natural killer (NK) cell-sensitive, experiments have investigated the influence of tumor immunology in metastases using this model (76). Earlier work has shown the AC inoculation approach producing metastases to the lungs (27,29,30). However, studies conducted in the past decades have not reported metastases (21-24). In addition, a study that used a suprachoroidal approach with this cell line in mice did not report metastases (23). The effect of NK cells on B16-F10 tumor metastasis remains controversial, with some authors stating it does not promote metastases, but others reporting NK-depleted mice have accelerated metastases formation (29).
The B16-F10 Queens melanoma cell line, with a higher metastasis formation rate, has also been inoculated into murine eyes (27). The timing of enucleation and sacrifice for metastases are comparable to the F10 cell line. Both suprachoroidal and AC inoculation methods have been described with great success in the formation of the primary tumor, but studies on metastases have been varied. The suprachoroidal approach showed metastases to the lungs (23,40). The AC approach has also shown to form pulmonary metastases, but extra-pulmonary metastases have not been consistently reported (25-27).
Though mice inoculated with Queens cells are favored for studying metastases formation over the B16-F10, the reliable primary tumor formation from both cell lines make them strong candidates to use in an animal model.
The HCmel12 melanoma is a mouse macrophage-attractive cutaneous melanoma cell line that has recently been introduced as a UM model in mice. Intravitreal injection of these cells into mice show choroidal melanoma formation and metastases to the lung and lymph nodes (70). Further, the cells demonstrate significant angiotropism and vasculogenic mimicry, which are seen in metastasizing human UM (73). A study has also reported the presence of a GNA11Q209L mutation variant, a driver of human UM, in the cell line (82). Additional studies with different inoculation methods are required to assess the cell line’s capability to study UM.
The rabbit eye has been used to study UM for decades because of its relatively large size, allowing routine examination of the posterior segment through fundoscopy and fundus photography. Ophthalmic surgery is much easier compared to the murine and hamster model and the anatomy of the rabbit eye is well-known. Furthermore, rabbits are relatively inexpensive to maintain compared to animals that have comparable sized eyes and have a longer lifespan than mice and hamsters (76).
The hamster Greene melanoma cell line was successfully grown in the rabbit eye. AC, subchoroidal, and suprachoroidal approaches have been described. Amelanotic Greene melanoma can be grown in the AC of rabbits, with rapid tumor growth and globe rupture documented 1–2 weeks after inoculation (Figure 4) (33). The same tumor can also be inserted into the subchoroidal space, demonstrating rapid growth of choroidal melanoma (92). Subsequent work has corroborated rapid tumor growth in the AC but did not document metastases in this method (34,35). A study injected cells into the subchoroidal space of rabbits, reporting formation of the tumor in 3–4 weeks and perforation of the eye in 6–8 weeks after subchoroidal implantation. After the rabbits were sacrificed, visceral metastases were noted, including those to the liver (64). Another study demonstrated the subchoroidal approach to reliably produce tumors, reporting presence of metastases to the rabbit kidney (65). The suprachoroidal technique has also been shown to produce primary tumors but was not studied for metastases formation (52).
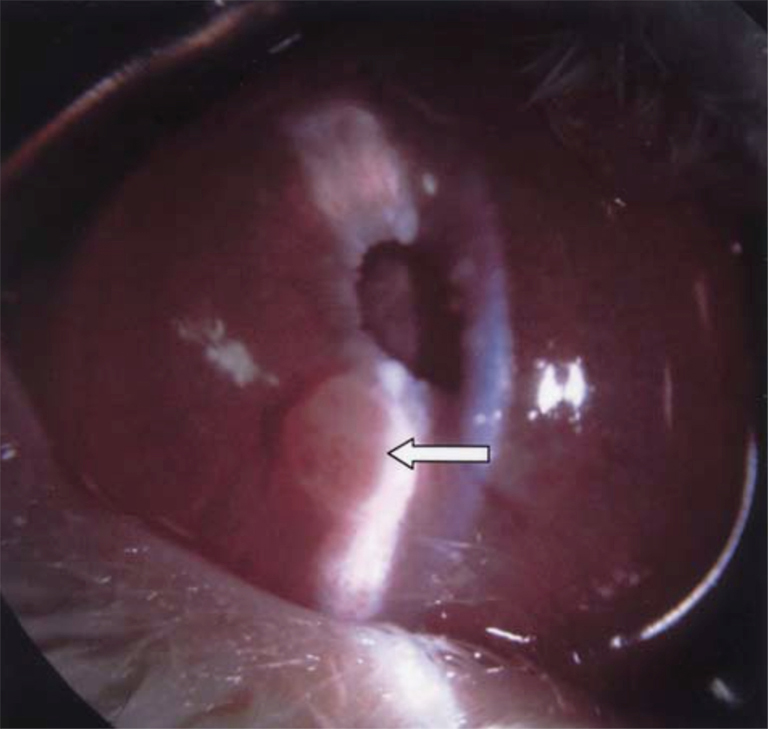
Perhaps the largest benefit of using this cell line in rabbits is the lack of immunosuppression requirement. However, its rapid growth and tendency for hemorrhagic necrosis limits its use in long-term study and metastases formation, as most animal models can only be examined for up to 2 weeks.
Even though the B16-F10 cell line is a mouse cutaneous melanoma, it has been inoculated into the rabbit with immunosuppression. AC and subchoroidal approaches with subconjunctival methylprednisolone and cyclosporin A, respectively, have been demonstrated to produce primary tumors (36,37). Transscleral inoculation of pieces of tumor from C57BL/6 mice can be grown in the rabbit subchoroidal space (66-68). These cells grow rapidly into heavily pigmented tumors and metastatic lesions are only found in the lung, consistent with the metastasis profile of the cell line (37). These studies were unable to consistently demonstrate extra-pulmonary metastases, though a rabbit model of extrascleral UM growth has been reported (93).
The advantage of this model includes its reliable primary tumor formation. A major disadvantage is the need for daily immunosuppression, whether that is corticosteroid application or cyclosporin A injections. This depletes the immune system of these animals to reduce rejection but tends to decrease their life span and possibility for infections in these animals (76). This makes this model unsuitable to investigate tumor immunology and metastases formation.
The inoculation of human cells into animals is becoming more widely used as they are recognized as more representative models to study human UM. Xenografts have the potential to elucidate the pathogenesis, metastases, and treatment responses for UM.
The athymic nude mouse has been a key xenograft model. With its immunocompromised state, these mice are unable to reject human donor tissue. Both AC and PC administration has been demonstrated. Niederkorn and colleagues have successfully transplanted OCM-1, OCM-3, and MEL202 cell lines into the AC of nude mice. They found tumor growth and necrotic liver metastases, deducing that these metastases halt growth after spread from the primary tumor (31). Another study administered 4 human UM cell lines into the AC of these mice and used various treatments to deplete NK cells. They found that the OCM-3 cell line was susceptible to NK-mediated cytolysis. By studying mice with and without NK activity, they found that NK cells play a role in combating UM metastases and that more mice injected with OCM-3 cells produced hepatic metastases (33). Inoculation of other human UM cell lines suprachoroidally have also shown to produce primary tumors and liver metastases (85). Another group injected UMT2 and UMT42 cells suprachoroidally, showing intraocular tumor growth in 100% and 25% of mice eyes, respectively, after 3 months. However, no metastases were detected (86). Ectopic xenograft mice models have also been used to study tumorigenesis on the subcutis of mice (83).
The primary advantage of xenograft mice is its high reproducibility, though they require immunosuppression (87). A significant shortcoming is that each cell line used in these models requires genetic validation to ensure it does not contain non-UM canonical mutations. This is critical for accuracy, as there have been reports of human UM cell lines that were found to be cutaneous in origin (88,89). Another disadvantage is the inherent genetic instability of UM cell lines cultured in vitro. Chromosomal analysis has shown that human UM cell lines differ from their parental tumor, even after passaging cells for 4 generations (90). These changes continue to increase as cells are propagated for a longer period (84).
To combat the limitations of culture-derived mouse xenografts, UM researchers have attempted to create patient-derived xenograft (PDX) mice. Direct tumor implantation is performed by inoculating fragmented human tumors without cell passage, producing UM that retains the original tumor’s genetic and histopathologic profile. These investigations for PDX are outlined in detail by Carita and colleagues (91). Murine PDX models offer a great avenue to explore tumorigenesis, explore experimental therapies, and emulate human UM metastases unlike any other model. Xenografts from metastases also have high tumor engraftment rates. The disadvantage of these animals is their high cost (9). Future studies are needed to form clinically relevant models with canonical UM mutations besides BAP-1 and investigate the potential of PDX and tumor organoids in this capacity.
Human cell lines have been used to develop an orthotopic model in rats using a suprachoroidal approach, as rats are not known to produce spontaneous UM (108). Three human UM cell lines have been used: OCM1, M619, and C918. Subcutaneous implantation of the human cell line OCM1 on the athymic albino rat produced skin melanomas, which were subsequently placed suprachoroidally in another rat. Researchers administered spheroids of human UM cell lines, which contained aggregates of human UM cells, suprachoroidally. These particles were able to colonize the subchoroidal space more effectively without contaminating the extraocular space. Choroidal melanomas in that study grew in almost all rats 4–41 days after inoculation (61).
This model is a great option to reliably study primary tumorigenesis in eyes that are larger than the murine eye. Given the human cell line, tumor behavior is likely similar to that of human UM. Tumors can be followed by high-frequency ultrasound reliably for up to 4 weeks and the animals do not display significant weight loss, which would require researchers to remove them from the study for ethical reasons (62). Angiogenesis and treatment options could also be studied in detail with this model. One shortcoming of the model is that the athymic rat lacks an immune system. Though this means there is no need for immunosuppression, it prevents the study of tumor immunology. Other disadvantages are that the model has not been widely used and has not been shown to produce metastases.
Several human UM cell lines can be used to study UM in rabbits. AC, suprachoroidal, and subchoroidal approaches have been shown to produce primary tumors. The human UM cell lines predominantly studied include OCM-1, MKT-BR, 92.1, and SP6.5. Kan-Mitchel and colleagues successfully grew human UM cells in the AC of rabbits, noting that cyclosporin A-treated rabbits show increased growth and survival of the AC tumors (38). Another group used the human ciliary body tumor-derived IPC227 cell line into the AC, reporting development of iris melanomas in all rabbits (39). No metastases were reported in studies examining tumors inoculated into the AC.
Among the four human UM lines, 92.1 and SP6.5 form choroidal melanomas with high aggressive local behavior in rabbits with suprachoroidal inoculation. They can also be used to study lung metastases, reported in 61% and 42% in the 92.1 and SP6.5 group, respectively (53). Suprachoroidal administration of OCM-1 cell pieces was highly successful in a study, reporting melanomas in 2 out of every 3 rabbit studied (54). Several studies applied the suprachoroidal approach to inoculate the 92.1 cell line, demonstrating lung metastases and liver micrometastases (55-57). However, work by other groups using the same method and cell line did not report metastases (58,59). In another study, implanted MKT-BR cells suprachoroidally, noting pulmonary metastases but not hepatic metastases (60). Subchoroidal placement of human UM cells in immunosuppressed rabbits has also been documented, with discontinuation of cyclosporin therapy showed regression of the tumor in 2 weeks (69).
Limitations of this model includes the need for immunosuppression and variable production of metastases. It has strengths in the study of primary tumorigenesis for choroidal melanomas. Future research needs to explore other cell delivery techniques and cell lines with higher rates of metastases, especially for AC inoculation.
GEMs comprise animals in which oncogenes can be constitutively or conditionally expressed and tumor-suppressor genes silenced using methods, such as retroviral infection, microinjection of DNA constructs, and the so-called “gene-targeted transgene” approach. GEMs capture both tumor cell‐intrinsic and cell‐extrinsic factors that drive de novo tumor initiation and progression toward metastatic disease. Genetically engineered mouse models have been the mainstay to study UM.
Since initial attempts to make transgenic mice from simian virus injections, great advances have been made using targeted gene expression approaches (9). Transgenic mice in modern research can be divided into two groups: Those that have a loss of function mutation and those that have a gain of function mutation. Among knockout mice, those with permanently inactivated target gene expression in every cell of the organism make up the ‘constitutive models’ while ‘conditional models’ are those with an inducible inactivation of gene expression. These can affect a specific target tissue or can occur in a time-controlled manner.
One of the first conditional models used to study UM had the integrated fusion gene containing the simian virus 40 (SV40) early region under the tyrosinase promoter expressed in melanocytes. In 1991, Bradl and colleagues produced C57BL/6 transgenic mice. Fertilized eggs were microinjected with 1 picoliter DNA, corresponding to 300 copies of the Tyr-SV40E transgene. The embryos were then transferred to the oviducts of the female mice. They observed that 14 of the 18 mice developed early melanomas from the retinal pigment epithelium. All the mice had locally aggressive disease, and 11 were found to have extra-hepatic metastases when sacrificed at 12 weeks (109). Subsequently, the human mutated Ha-Ras (TPras) transgenic mice model was created using mouse tyrosinase promoter sequences to drive expression of the mutated human Ha-ras gene. This transgene contains a tyrosinase promoter fragment, which can be ligated into a promoterless construct containing the mutant T24 c-Ha-ras gene in the pIC-20R vector. These models show rapid growth of melanomas, but tumors originate from the retinal pigment epithelium (RPE) and not the uvea (110,111). Furthermore, these mice did not show evidence of metastases (110). Subsequently, transgenic mice expressing the human mutated Ha-ras with lnk4a/Arf-deficient backgrounds showed UM with similar histopathological features to human UM (112).
Other oncogenes like RET have also been commonly utilized in the formation of these models. The metallothionein (MT)-RET.AAD transgenic mouse harbors the combination of RET and the chimeric major histocompatibility complex molecule AAD (alpha1-alpha2 domains of HLA-A2 linked to alpha3 domain of H2-Dd). The RET oncogene is conditionally expressed by melanocytes, leading to UM formation and melanogenesis in additional tissues (113). Vector formation and administration are similar to the other transgenic models described, in which the RET plasmid fragment is injected into the eggs and implanted into females (114). These mice showed choroidal UM, with 93% of mice showing exophthalmos due to UM by 150 days of age and 47% within 30 days of birth (114). Another study found that melanomas in RET.AAD transgenic mice were reproducible only in the choroid or ciliary body. Distant metastases were observed, with tumors detected in the reproductive tract at a median age of 242 days, the mediastinum at a median age of 263 days, and the lungs at a median age of 347 days (115).
In recent years, additional signaling pathways were established in the pathogenesis of UM. As the metabotropic glutamate receptor 1 (GRM1) is expressed in human UM, the glutamate signaling pathway has been studied in transgenic mice. These mice have an insertion of the transgene into an intron of GRM1 and its conditional expression is under the melanocyte‐specific dopachrome tautomerase promoter. Researchers have shown these mice exhibited pigmented choroidal proliferation and UM-like tumors, mimicking spontaneous UM and opening possibilities for therapeutic intervention. At 7 to 8 months, mice developed choroidal thickening with positive S100 and Melan-A markers, uveal melanocytic neoplasia, and numerous Ki-67-positive cells in the choroid. However, they were unable to detect metastases in their model (116,117).
One of the main drawbacks of using transgenic mice is that the melanomas do not reliably originate in the uvea and longer times are required to generate new mice. Furthermore, the molecular changes reported are not consistently observed in human UM. Tumors are unable to show hepatic metastases unless cells are transferred intracamerally and a substantial fraction of mice can also exhibit heterogeneity in their phenotypes. Other transgenic mouse models include TySV40, Tyr-Tag, and TRP-1/Tag (118-120). The features of these models, in addition to ones discussed here, are highlighted in Table 3.
| Mouse model | Model features | References |
|---|---|---|
| TySV40 | Bilateral intraocular tumor developed and metastasis was found in the liver | (118) |
| Tyr-SV40E | Mice developed ocular and cutaneous melanomas. Diffuse metastasis to various internal organs was seen | (109) |
| RET.AAD | Pigmented tumors were first observed in the choroid and ciliary body. Metastasis were found in various internal organs | (115) |
| Tg (Grm1) EPv | Pigmented choroidal tumors developed in the mice. Hepatic metastases were noted | (117) |
| Tyr-Tag | Mice develop intraocular tumors similar to human UM. Metastasis was observed in subcutaneous tissue, lung and brain | (119) |
| TRP-1/Tag | Tumors of pigmented and epithelial cell origin developed in the mice and metastasis was detected in the spleen and inguinal lymph nodes within 3 months | (120) |
UM, uveal melanoma; TRP-1, tyrosinase-related protein 1 promoter.
Induced models focus on physical and/or chemical stimuli for UM formation. Physical stimuli such as irradiation, may be used individually or in combination with chemical stimuli such as viruses or genetic constructs. Induced models have gained recognition in the recent years due to ease and availability of various protocols and techniques. Further, unlike transgenic models that may cause a shortened lifespan, this approach can be used to grow UMs at different sites at various stages of development in animals (121).
Several methods of mutant induction are used to generate animal models of UM, some of which are highlighted in Table 4. Chemical and radiation-induced mutations were among the first methods used to create induced UM. Although these methods resulted in intraocular tumors, it did not lead to reproducible animal models (73). The main advantages of this method were the low cost of induction and the high mutational rate seen in the animals. However, the uncontrolled carcinogenesis results in increased lethality and little control over experiments, making these methods less suitable for studying UM.
| Induction method | Advantages | Disadvantages |
|---|---|---|
| Chemical/radiation induced mutations | High mutational rate; minimal cost for induction of mutation | Random integrative mutations; difficult to associate specific mutations with pathologies |
| Retroviral infection | Insertion of specific gene; low controlled events | De novo DNA methylation; vector capacity in large genes; random integration in the genome |
| Microinjection of DNA constructs | Direct insertion of specific gene | Random integration in the genome |
Retroviral DNA insertion stands among the first attempts to generate “gene-trap” approaches for transgenic mice (121). This method involves infecting embryos or specific tissues with recombinant viruses carrying the engineered gene of our choice but under the control of its own promotors. The control of tissue specificity can be achieved by incorporating into the lentiviral vector promoters specific for certain tissues. The advantage of this method is that a single transgene of interest can be delivered to the tissue, which can be transferred to progeny. De novo DNA methylation that influences the expression of viral genes can be a limiting factor, along with the restricted capacity of the vector to carry the transgene.
Microinjection of DNA constructs is another procedure which had undergone many modifications over the years. The aim is to develop a strategy to create a mouse engineered to express a specific target gene of interest (122). The general principle of DNA microinjection technique consists of preparing a construct carrying a transgene and a collection of one-cell fertilized embryos, followed by direct injection of the construct into the embryos. The gene-targeted transgene method in another way to induce mutations which involves targeted modifications of mouse embryonic stem (ES) cells collected from the inner cell mass of E3.5 blastocysts (123). Like retroviral infection methods, these methods also involve the risk of random insertion into the genome (124).
Finally, recombination and targeted deletion of genes is one of the most powerful methods used to study UM. Researchers can introduce mutations from a single base pair to megabase pairs at the chromosomal level. Cre-mediated recombination is widely used for conditional gene deletion in a specific tissue, as the Cre recombinase of phage P1 can mediate excessive gene recombination in mammalian cells between loxP sequences (125). Injection of the recombinase via adeno-associated virus (AAV) into the choroid of transgenic mice carrying a double floxed gene has been successfully demonstrated. This method can allow the study of genes implicated in UM tumorigenesis through elimination of key regulators in signaling and tumor growth.
After the discovery of GNAQ and GNA11 oncogenes as the main cause of UM, several mouse models have been developed using these genes. The first attempt at this was by Feng et al who generated a mouse model expressing HA-GαqOL under the control of the tet-responsive elements (tet-HA-GαqOL) where more than 50% of mice developed cutaneous melanomas but there was no report of uveal tract lesions (126). Another group successfully introduced the first induced mouse model of UM, which consisted of GNAQQ209L overexpression under the Rosa26 promoter. The vector was transfected via electroporation of hybrid embryonic stem (ES) cells, which were injected into C57BL/6 blastocysts. All 15 mice developed UM at 3 months with melanocytic tumors found in the lungs and meninges (127). Subsequently, Moore and colleagues generated another mouse model with melanocyte-specific expression of GNA11Q209L with and without homozygous BAP1 loss. The addition of BAP1 loss increased tumor proliferation and melanoma size. At 6 months, almost 50% of the mice developed UM, with metastasis to axillary lymph nodes and lungs in all mice (128).
Unlike previous attempts, where the broad Cre expression in extraocular melanocytes led to early lethality and complicated UM phenotypic analysis, Li and colleagues adapted an AAV-based ocular injection method to directly deliver Cre recombinase into the uveal tract. They delivered the AAV5-CMV-Cre viral vector into the choroid and, subsequently, delivered Cre recombinases into adult mice carrying the floxed alleles for both Lats1 and Lats2 genes that drives the expression of membrane-bound GFP proteins upon Cre recombination. Mice showed proptosis in 2 months, and 8 out of 10 mice developed UM with no sign of extra-ocular growth in 6 months (129).
Researchers developed a feline model of UM to determine aspects of the natural history and immunology of UM. They inoculated the Gardener strain of the feline sarcoma virus (FeSV) at the inferior root of the iris and observed that tumors developed in 90% of inoculated eyes, demonstrating hypertrophy, hyperplasia, and atypia of the uveal melanocytes. Fourteen of the 36 cats showed signs of extraocular spread to orbital muscles, skeletal muscle, pleura, and pericardium (130).
Albert and colleagues injected nickel subsulfide (Ni3S2) into the vitreous cavity of rats and observed the development of intraocular UM 6 to 9 months later. Electron microscopy of the tumors demonstrated premelanosomes, but no metastases were reported (63).
Researchers induced primary uveal melanocytic lesions in Dutch rabbits. Their protocol involved four-weekly topical applications of dimethyl-benz[a]anthracene (DMBA) in acetone followed by 12 weekly topical applications of 10-μL solutions of either 0.25% or 0.5% croton oil in acetone. Exposure to DMBA, followed by promotion with croton oil in either concentration, was an effective means of inducing choroidal melanomas. However, no metastases were reported (131).
Murine and rabbit xenograft models may have limited utility for screening large compound libraries in drug discovery studies, necessitating new preclinical models. Zebrafish (Danio rerio) has been commonly used for these investigations. Ent and colleagues used transgenic zebrafish expressing enhanced green fluorescent protein in endothelial cells to inject human UM primary tumor cells (92.1, Mel270) and cells from human UM metastases (OMM2.3, OMM2.5, OMM1). Approximately 400 to 500 cells were injected into the yolk sac of zebrafish using a pneumatic pump. Automated confocal image analysis showed cellular proliferation and active migration. Using in vivo imaging coupled with 3D reconstruction, interactions between these cells and the external surface of zebrafish vessels were successfully documented (6). Advantages of these animals include low housing costs, easy manipulation, a large clutch size, and the benefit of ex utero development.
Chick embryo is another model has been used successfully to study UM. Cells can be implanted onto the chorioallantoic membrane (CAM), delivered intravenously, or placed into the optic cup. Human metastatic UM cells injected into 20 day 4 embryos demonstrate 4 of 18 surviving embryos with intraocular UM on day 19. Metastatic UM cells can also be found on the ciliary body along the hyaloid artery and the tunica vasculosa lentis, suggesting dissemination from the uvea (132). Other researchers have developed CAM assays to study UM. In these assays, UM cells are engrafted onto the CAM through a small window in the shell of the egg without damaging embryos. They labeled OMM1 and 92.1 cells with green fluorescent protein and injected them into the developing eye of these embryos on day 7. They found that the cells homed to the uveal tract on day 14 (Figure 5). Tumors rapidly grew in half of the embryos 7 days after inoculation, forming nodules measuring up to 1 cubic millimeter in 10% of all animals in their study (133). Other investigators have reported successful outcomes with other human UM cell lines, as well (134). Some advantages of this model are the ability of UM cells to undergo orthotopic growth in the chick eye, form tumor masses on the CAM, and undergo dissemination via the chick circulation to internal organs.
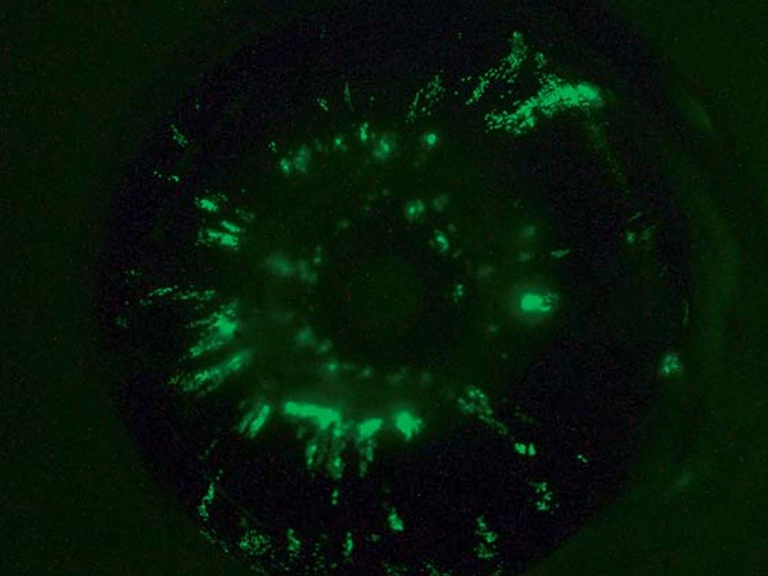
Approximately half of patients with UM suffer from metastatic disease. As such, animal models that allow for the study of visceral metastases has been imperative. Mice have been used as hosts for this purpose, with non-human cutaneous or human UM cells introduced either into visceral organs or directly into the systemic circulation. Cell lines from human metastatic UM are in use and their origins described elsewhere (84). Intrahepatic injection or implantation, splenic injection, intracardiac delivery or direct intravenous injection via the tail vein have been described (43,106,135,136). One group developed a mouse model with EGFP-luciferase-labeled human OMM1.3 cells into the retroorbital space of mice with SCID. They observed pulmonary and hepatic metastases in 6 to 7 weeks (137).
Orthotopic PDX models from hepatic metastases, in which patient metastases are implanted into the liver of NSG mice, have also been investigated (138). One study reported a tumor engraftment rate of 83% with this method, with the ability to follow tumors by computed tomography (107). Another study showed that splenic injections of patient-derived metastatic UM cells formed tumors diffusely throughout the liver compared to the hepatic injection that formed a single tumor (136). Additional treatments for human metastatic UM with radiotherapy, chemotherapy, gene therapy, and other agents have been explored (4). Details of metastatic UM models and treatment strategies are discussed elsewhere (4,73,139).
These models offer the study of metastases in an isolated fashion but limit its generalizability to human UM as a result. A metastatic UM model that recapitulates dissemination from a human intraocular UM is still a work in progress.
UM is a rare disease studied for decades, but there is still much to discover. Compared to many cancers, research in the field has translated into minimal clinical benefit for patients. Animal models continue to represent platforms to elucidate primary tumor growth, mutagenesis, metastasis, and immunobiology of UM. Models can be created with different inoculation and cell lines, with unique benefits and limitations. Engrafted models are strong candidates to study tumorigenesis but are cutaneous in origin. Animal and patient-derived xenografts recapitulate human UM but require immunosuppressive treatment. Transgenic models are excellent in researching primary UM in otherwise healthy hosts but have variable metastatic profiles. Animal models for metastatic UM allow for the focus on metastases but require surgical expertise and lack primary tumors. The “best” model is the model that answers the research question, so knowledge of the advantages and disadvantages of each model is key.
The transformational value of preclinical UM studies remains limited by the differences in molecular and biological characteristics of UMs in animals and humans. Incongruences in tumor environments and immune properties further complicate this matter. Scientists continue to bioengineer animal models where tumors will behave similarly as in the human body. In the future, we will likely see additional orthotopic PDX models that closely mimic human UM. Similarly, techniques to increase the engraftment and mutagenesis rates will be developed. Models for metastatic UM will likely be improved to study metastasis evolution. As exciting models continue to be explored, novel strategies are needed to study human UM to ultimately present therapies that improve patient survival and quality of life.