Abstract: In a comprehensive literature review, PubMed, Embasem and Web of Science were searched for studies examining targeted therapy of ocular malignant melanomas to present and discuss targeted therapy treatment options of ocular tumors, mainly conjunctival and uveal melanoma (UM). Conjunctival malignant melanomas showed similarities in clinical and genetic aspects with cutaneous melanomas. Many therapies with checkpoint inhibitors already established for cutaneous melanomas may be a treatment option for conjunctival malignant melanomas with shared traits. Existing targeted therapies are for example checkpoint inhibitors like pembrolizumab or nivolumab. As a corollary, due to marked differences in clinics and genetics between UMs and conjunctival melanomas (CMs) or cutaneous melanomas, it has remained elusive whether the available possibilities of molecular targeted therapy will be an option for the therapy of metastasizing UMs. Possible novel ways of treating UM are being explored. Fotemustine or the inoculation of dendritic cells with tumorous RNA or sunitinib in combination with cisplatin and or tamoxifen may be used in future to treat UM. While CM are treatable using targeted therapies, UM have not been researched enough to find working targeted therapy options. Further research has to be done in order to find acceptable treatment options.
Targeted therapies or molecularly targeted therapy of malignant tumors have become a pillar of the treatment of malignancies in the last decade (1-3). A targeted molecular therapy is a treatment option on the molecular level, blocking the growth of cancer cells by interfering with specific targeted molecules. The molecules are needed for carcinogenesis and growth of the tumors rather than by simply interfering with all rapidly dividing cells in the body as it is achieved by conventional chemotherapy. Besides hormonal therapy and cytotoxic chemotherapy, targeted molecular therapy is one the three types of pharmacotherapy for cancer. Using highly specific molecules affecting the cancer-related processes in a focused manner, side effects caused by systemically applied cancer drugs can be markedly reduced while, simultaneously, the more precise and effective targeting of the tumor cells may improve the outcome. The purpose of this review is to summarize the potentials of a targeted therapy for primary ocular malignant tumors in adults. These tumors mainly include conjunctival melanomas (CMs) and uveal melanomas (UMs), which can be sub-differentiated into melanomas of the ciliary body, the iris and the choroid.
Using electronic bibliographic databases, PubMed, Embase, and Web of Science were searched for the following keywords with different combinations: “ocular melanoma”, “targeted therapy”, “uveal melanoma”, “conjunctival melanoma”, “mitogen-activated protein kinase (MAPK)”, “PI3K/mTOR”, “BRAF V600E mutation“, “BRAF-mutated conjunctival melanoma”, “pembrolizumab”, “nivolumab”, and “checkpoint immune therapy”. Searches were limited to English and German human studies until May 16th, 2020.
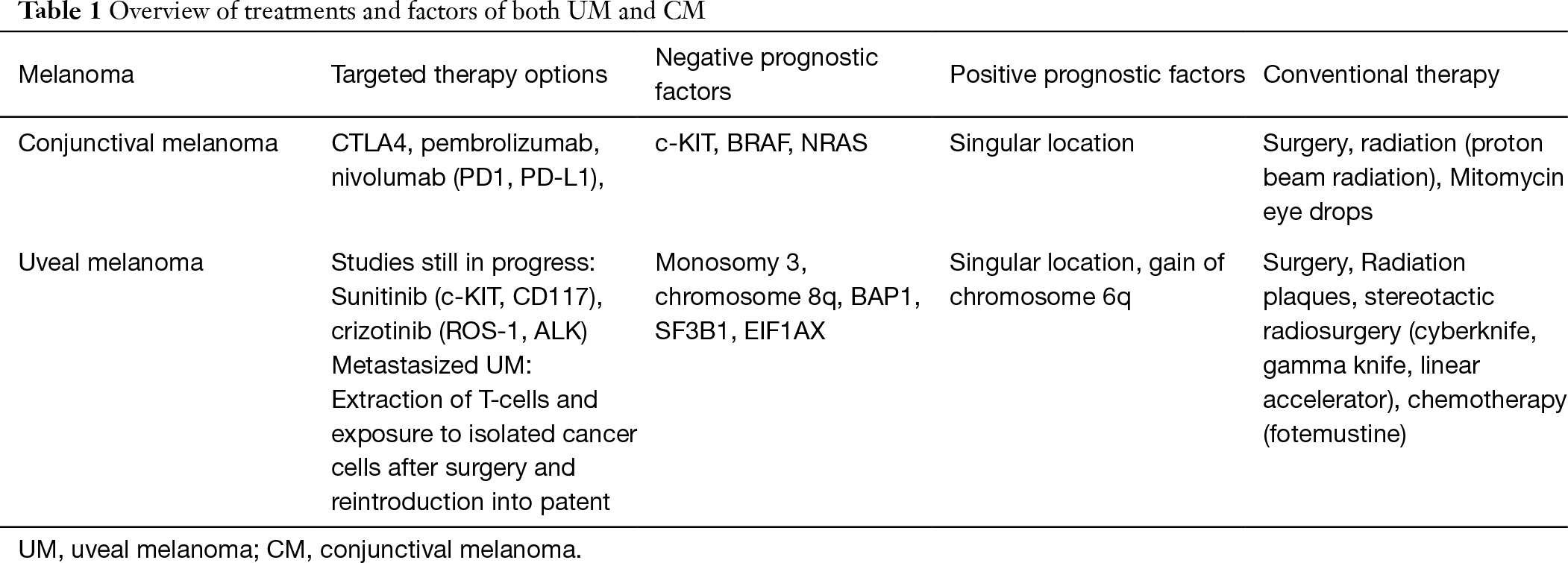
CMs, malignant melanocytic lesions occurring on the ocular surface, have a low prevalence but showed an increase in their incidence in Europe and the United States in the last decades (4-8). Its prevalence in the United States and Europe was estimated to be approximately 0.5 to 1.0/million (4). After surgical excision CMs frequently recur locally with a frequency estimated to range between 30% to 60% and can lead to lethal metastasis (9-13). Options for systemic therapy of tumor-distant metastases have been limited and lead to death due to metastasis in about 10% to 35% at 10 years follow-up (9-13). CMs share many similarities with cutaneous melanomas including lymphatic metastasis, clinical characteristics, and molecular genetic pattern. As in the case of cutaneous melanomas, the mutation load is high in CMs with about 90,000 mutations in the entire genome, and most mutations in CMs are cytosine to thymine transitions, potentially as a sequel of ultra-violet light-induced damage (14-16). UMs usually show a markedly lower mutational load, also with different mutations involved, and the clinical features of UMs differ profoundly from those of CMs. CMs as well as UMs have been discussed to represent different types of cancer (17). As a corollary, mutations commonly observed in cutaneous melanoma, like V600E in exon 15 of BRAF, Q61L in exon 3 of NRAS or NF1 mutations, have also been detected in CMs. BRAF mutations were detected in 29% to 35%, NRAS mutations in 18%, and NF1 mutations in 33% in CMs (15,18-22).
A precursor for CMs is usually a primary acquired melanosis or a conjunctival melanocytic nevus, while only rarely a CM develops de novo (8,23). Clinical features include a brownish pigmented, slightly elevated lesion in the conjunctiva with some hyperemia and dilatation of the feeding conjunctival vessels. Few studies reported also amelanotic CMs (24-27). CMs in their early stage can be misdiagnosed as a therapy-resistant conjunctivitis (28). CMs can grow aggressively and spread superficially on the conjunctiva and extend onto the corneal surface, as well as can grow locally invasively into the deeper tissues. Diagnosis of CMs should include a photographic documentation of the lesion, sonographic examination of the eye and search of metastases (29).
The therapy of CMs includes the excisional “no-touch” biopsy of the suspicious lesion, followed by a histopathological examination to assess the nature of the lesion and whether the resection edges are free of tumor cells. An analysis of the serum concentration of lactate dehydrogenase and S100 can also be performed. S100 are CM tumor markers indicating potential CM metastases. A genetic analysis of the samples should search for c-KIT (tyrosine-protein kinase KIT, CD117) and BRAF (proto-oncogene B-Raf), and if not found then NRAS (neuroblastoma RAS) (29,30). Staging using the staging manual of the American Joint Committee of Cancer (AOJCC) and the final diagnosis consists of searching for malignancies in the oropharyngeal compartment and checking of the lymph nodes of the head and neck (29,31). The surgical excision of the primary tumor is followed by adjuvant therapy such as brachytherapy, proton beam radiation, or topical medical therapies such as the application of mitomycin eye drops (32-34).
If metastases have developed and with no possibilities for a curative surgical treatment, targeted molecular therapies applying checkpoint-inhibitors such as pembrolizumab or nivolumab come into play. As cancer immunotherapy, checkpoint inhibitor therapy targets immune checkpoints, which are key regulators of the immune system. Activation of these immune checkpoints increases, and inhibition decreases, the immune response to an immunologic stimulus by changing the degree of the T-cell activation or the T-cell effector function. Physiologically, the inhibition of the immune checkpoints serves to prevent the development of auto-aggressive diseases. By stimulating inhibitory immune checkpoint targets, some cancers can protect themselves from an immunological attack by T-cells. A checkpoint therapy blocks inhibitory checkpoints and thus restores the normal function of the immune system. Checkpoint inhibitors target currently approved in medicine include the molecules CTLA4, PD-1, and PD-L1. PD-1 is the transmembrane programmed cell death-1 protein (also called PDCD1 and CD279), which interacts with PD-L1 (PD-1 ligand 1, or CD274). PD-L1 on the cell surface binds to PD1 on an immune cell surface and thus inhibits the immune cell activity. An important function of PD-L1 is the regulation of T-cell activities, so that a cancer-mediated upregulation of PD-L1 on the T-cell surface may inhibit T-cells and prevent them from attacking the cancer cell. Inactivation of PD-1 or PD-L1 by antibodies binding to either of them block their interaction with the T-cell and may thus allow the T-cells to attack the tumor.
Pembrolizumab or nivolumab are currently discussed for the checkpoint-associated therapy of metastasizing CMs, with small case series being published (34-39). However, results of a prospective randomized treatment trial are missing yet. It has remained unclear how far results of phase-III trials on the therapy of cutaneous melanomas using checkpoint inhibitors can be directly transferred on the therapy of CMs (23,40-43).
Uveal malignant melanomas, representing about 5% of all malignant melanomas, can be located in the choroid (90%), ciliary body (6%), or iris (4%), and have a mean age-adjusted incidence of 5.1 cases per million per year in Caucasians. They are the most common primary intraocular tumor in adults (44-51). UMs usually manifest in the sixth decade of life. Risk factors include fair skin, blue iris color, inability to tan, ocular or oculodermal melanocytosis, cutaneous or iris or choroidal nevus, and BRCA1-associated protein 1 mutation. Currently, the most widely used first-line treatment options for this malignancy is a local resection, either as a transscleral resection or by an intraocular approach, radiation therapy, and enucleation. Radiation treatment is differentiated into plaque brachytherapy using plaques loaded with iodine-125, ruthenium-106, palladium-103, cobalt-60, and tele-therapy applying proton beam therapy, helium ion therapy, or a stereotactic radiosurgery such as cyber knife, gamma knife, or linear accelerator. Despite all research and improvements in the diagnostic capabilities, the long-term survival rate has remained guarded and mostly unaffected by the therapy. In contrast, the possibilities for a globe and vison salvaging therapy have markedly improved by the increased surgical and radiological treatment options. UMs metastases usually occur in the liver by hematological pathways (44-54).
Despite similarities in their name and stemming from melanocytes, UMs and cutaneous melanomas (and CMs) show profound differences in their risk factors, clinical characteristics and course, metastasizing, genetic pattern and molecular changes, and responses to systemic therapy including targeted molecular therapy. If metastases of UMs have occurred, the life expectancy is markedly reduced, and therapy options are rather limited. It has to be stressed that the characteristics, therapy options and prognosis differ profoundly between CMs and UMs, so that extrapolations from cutaneous melanoma therapies to the treatment of UMs are not possible (44-54).
Although no evidence-based therapy for metastases of UMs is available yet, prognostication is important for counselling of the patients and planning of follow-up examinations. Besides conventional clinic-pathologic characteristics, including size and location of the tumor and histological tumor cell type, genetic factors are of profound importance for the prognosis. Non-random chromosome aberrations such as monosomy 3 and gain of chromosome 8q are strongly correlated with the risk of metastases, while gain of chromosome 6p is associated with a low risk. In addition, mutations in genes like BAP1, SF3B1 and EIF1AX have been reported to be associated with the prognosis (45-52).
Patients with an UM can have a five-year survival rate of 80%. In dependence of high-risk genetic patterns, such as monosomy 3 and gain of chromosome 8q, the risk for the development of metastasis and the general prognosis can be markedly guarded. Metastases of UM show a high affinity to the liver, as it is commonly the first metastasis detected. Approximately 25% of all UMs develop metastases after 5 years and 34% after 10 years after local treatment. Metastases occur mainly in a hematogenous way, while conjunctival or skin melanomas metastasize rather lymphogenous.
There is no effective adjuvant therapy for metastases of UMs at the moment, while studies of innovative treatment regiments are ongoing, including clinical studies using the chemotherapeutics fotemustine; dendritic cells loaded with autologous tumor RNA to activate CD4- and CD8-T-cell response against tumor antigens; the kinase inhibitor sunitinib alone or in combination with cisplatin/tamoxifen; and anti-receptor tyrosine kinase drugs such as crizotinib (55-61). In contrast to skin melanomas, UMs have not been effectively been treated by targeted molecular therapy (62) (Table 1).
In conclusion, since CMs show marked similarities in clinical and genetic aspects with cutaneous melanomas, and since, systemic therapies with checkpoint inhibitors have already been established for cutaneous melanomas, the application of checkpoint inhibitors are a treatment option for metastatic CMs. As a corollary, due to differences in clinics and genetics between UMs and CMs or cutaneous melanomas, including the lack of lymphatic vessels in the eye, it has remained elusive whether the available possibilities of molecular targeted therapy are an effective therapy option for metastatic UMs (63,64).