Abstract: Our increase in knowledge of the pathophysiology of non-infectious uveitis (NIU) and other immune-mediated diseases has been mirrored over the last two decades by the expansion of therapeutic options in the realm of immunosuppressive medications. Principal among these advances is the emergence of biologics, which offer the promise of targeted therapy and the hope of reduced toxicity when compared to corticosteroids and “standard” immunosuppression. Among the biologics, monoclonal antibodies blocking tumor necrosis factor alpha (TNF-α) have been shown to be a very effective therapeutic target for uveitis and many associated systemic inflammatory diseases. Multiple TNF blockers have shown benefit for uveitis, and in 2016, adalimumab became the first biologic and non-corticosteroid immunosuppressive to obtain Food and Drug Administration (FDA) approval in the treatment of NIU. Although effective, TNF blockers are not universally so, and safety concerns such as infection and demyelinating disease must be carefully considered and ruled out prior to their use, especially in patients with intermediate uveitis with which multiple sclerosis is a known association. Ongoing study has identified novel targets for regulation in the treatment of immune-mediated and inflammatory diseases. Interferons, interleukin and Janus kinase inhibitors in addition to antibodies targeting T cell and B cell activation highlight the expanding field of treatment modalities in NIU. Ongoing study will be required to better determine the safety and efficacy of biologics in the armamentarium of immunosuppressive treatments for NIU.
Uveitis is characterized by intraocular inflammation affecting the uveal structures of the eye, including the iris, ciliary body, and choroid. This term can also be used to encompass inflammation affecting any intraocular structure, including the retina, retinal blood vessels, vitreous and optic nerve. The classification of uveitis may be based upon the location of the inflammation (anterior, intermediate, posterior or panuveitis) or the etiology of the inflammation (non-infectious or infectious) (1). Non-infectious uveitis (NIU) can manifest as a primary ocular disease or can be associated with many disparate systemic illnesses. A diagnosis of NIU is made after a thorough work-up which includes ruling out infectious and malignant etiologies, as well as assessing for known secondary causes of inflammation. This is of the utmost importance since the immunosuppressive therapies used in the management of NIU can have deleterious effects if used in the incorrect setting.
Recurrent uncontrolled flares of inflammation in NIU can result in a saw-tooth deterioration of the ocular anatomy and physiology and can subsequently cause permanent visual loss and blindness. Treatment goals therefore should aim to provide long-term steady control of the inflammation whilst minimizing the long-term side effects of therapy.
The general therapeutic algorithm for NIU involves the initial use of corticosteroids (topical, periocular, intravitreal or systemic) followed by the use of the ‘conventional’ systemic immunomodulators, which includes antimetabolites, calcineurin inhibitors and less commonly cytotoxic agents. Although there are now a few biologics that can be considered as first-line agents in the treatment of NIU, in the majority of conditions they are 2nd or 3rd options. This review will touch upon past and current biologics used in the treatment of NIU but will also discuss the promising newer therapies coming through—including anti-tumor-necrosis factor alpha blockers, inhibitors of T and B cell activation, interferons, interleukin inhibitors and Janus kinase inhibitors.
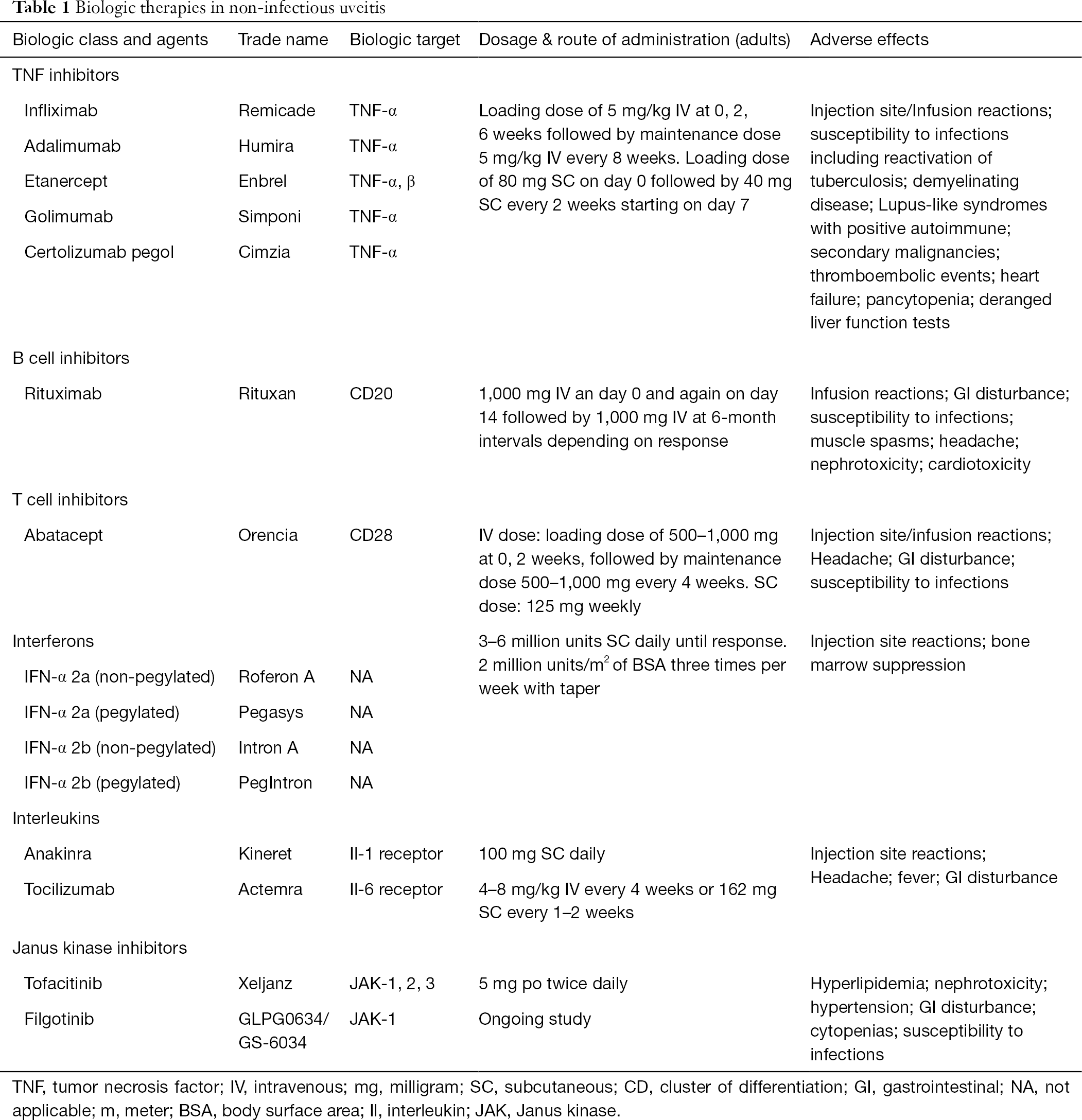
A biologic is a pharmaceutical drug that is either manufactured, extracted from, or semi-synthesized from a biologic source. The use of biologics as therapeutic options for NIU has increased over the past decade, mirroring the increase of their use for other systemic inflammatory diseases. Biologics used in uveitis come in a variety of forms, including monoclonal antibodies, bioengineered receptor complexes, cytokine antagonists, or cytokines—all with their own specific targets aimed to stifle and modulate the function of the immune system. Table 1 provides a summary of the various biologics that may be used in NIU, their dosing and potential adverse effects.
TNF-α is a pleiotropic cytokine that exists as either a membrane-bound or soluble cytokine—both of which are biologically active. It is secreted by various immune and non-immune cells and acts as an important inflammatory mediator in the body’s normal response to tissue injury and infection. TNF-α has been implicated as a key player in the initiation and propagation of inflammation in numerous inflammatory diseases. The downstream effects of TNF- α involve the rapid induction of cytokines such as Il-1 and Il-6 and the activation of Th1 CD4+ T cells (2). Targeting this cytokine has proven to be effective in the control of various systemic and ocular immune-mediated conditions. Specifically, in the treatment of NIU, the anti-TNF-α agents have evolved into both an alternative and synergistic option to the commonly used immunomodulatory agents. Biologics targeting TNF-α can be divided into two main groups: monoclonal antibodies that bind to and neutralize TNF-α (infliximab, adalimumab, certolizumab, and golimumab) and soluble TNF receptors that bind TNF-α (etanercept).
Infliximab (Remicade, Janssen Biotech Inc., Horsham, Pennsylvania, USA) is a chimeric mouse-human monoclonal antibody against human TNF-α. Once infused it binds to TNF-α and prevents it from binding to TNF-α receptors thus preventing the signaling cascade. It is not approved by the Food and Drug Administration (FDA) for treatment of NIU, but is approved for the treatment of rheumatoid arthritis (RA), inflammatory bowel disease, psoriasis, psoriatic arthritis and ankylosing spondylitis (3). Infliximab is administered as an infusion starting with a loading dose at 0, 2 and 6 weeks followed by maintenance doses that are usually administered every 4 to 8 weeks. The dose is typically adjusted depending on the condition being treated and the response to treatment (3).
The earliest evidence of infliximab being used to treat ocular inflammation came as early as 2001 where it was successful in treating a case of panuveitis and another of RA-associated scleritis (4). A number of case reports, case series and non-comparative studies have displayed the effectiveness of infliximab in the treatment of uveitis associated with HLA-B27 (5-7), juvenile idiopathic arthritis (JIA) (8), Crohn’s disease (9) and sarcoidosis (10,11). Infliximab has also been investigated in the treatment of uveitis associated with Behcet’s disease (12,13). One such study looked at the long-term efficacy of infliximab in the treatment of 164 consecutive patients with Beh?et’s disease treated for more than 1 year. Infliximab was successful in reducing the frequency of yearly flares and there was an improvement in BCVA in almost all of the studied groups (13).
Adalimumab (AbbVie Inc., North Chicago, IL, USA), a human monoclonal antibody targeting TNF-α, became FDA-approved for the for the treatment of RA in 2002 and has subsequently been approved for the treatment of JIA, inflammatory bowel disease, plaque psoriasis, hidradenitis suppurativa, psoriatic arthritis and ankylosing spondylitis (14). It currently the only FDA-approved biologic used in the treatment of NIU. It is administered through subcutaneous injection with a loading dose of 80 mg at week 0, a dose of 40 mg at week 1 followed by maintenance doses of 40 mg given at 2 week intervals thereafter.
Numerous published cases and studies have shown the effectiveness of adalimumab in the treatment of uveitis associated with JIA (15-17), Behcet’s disease (16,18), Vogt-Koyanagi-Harada (VKH) (16), sarcoidosis (16), ankylosing spondylitis (16), birdshot chorioretinitis (16), pars planitis (16) and idiopathic uveitis (16). The VISUAL trials were phase 3 multicenter, randomized controlled trials (RCTs) that were instrumental in establishing the safety and efficacy of adalimumab in the treatment of NIU (19-21). The VISUAL 1 trial recruited 223 patients with non-infectious intermediate, posterior, or panuveitis who still showed signs of active inflammation despite receiving oral prednisone at a dose of 10–60 mg daily for 2 or more weeks. At study onset, patients were required to undergo a 2-week prednisone burst of 60 mg daily followed by a protocol-mandated taper over the course of 15 weeks. The primary efficacy endpoint was time to treatment failure—a multicomponent outcome which included two-step worsening of anterior chamber cell grade, vitreous haze, new inflammatory chorioretinal lesions, and best corrected visual acuity (BCVA). The VISUAL 1 trial showed that patients taking adalimumab had a median treatment time to failure of 24 weeks compared to 13 weeks in the placebo group (19). At the 6-month time-point there was a 50% reduction in the risk of treatment failure in patients taking adalimumab compared to those who received placebo (hazard ratio =0.5; 95% CI, 0.36–0.70; P<0.001).
A similar study was designed to evaluate the efficacy of adalimumab in preventing uveitic flares in patients with inactive but steroid-dependent non-infectious intermediate, posterior and panuveitis. In the VISUAL 2 trial (20), enrolled patients were required to have no active inflammation on 10–35 mg of prednisone for at least 4 weeks. After randomization, subjects were subjected to a prednisone taper starting on week 2, which was completed no later than week 19. This trial showed that adalimumab was effective in preventing flares in this population, as the median time to failure was 8.3 weeks for the placebo group but since more than half of the adalimumab treated group did not experience treatment failure at the 18 month time-point, a median time for this group was not able to be estimated. There was a 43% reduction in the risk of treatment failure in the adalimumab group compared to patients on placebo (hazard ratio, 0.57; 95% CI, 0.39–0.84; P=0.004).
The VISUAL 3 study was an open-label extension trial that enrolled patients from VISUAL 1 & 2 with active [242/371 (65%)] and inactive [129/371 (35%)] non-infectious intermediate, posterior and panuveitis (21) after they either met a primary study endpoint or completed 18 months in one of the parent studies without a flare. Enrolled subjects all received open-label adalimumab at 40 mg every two weeks, and prednisone tapering and other immunomodulatory therapy occurred at the discretion of the investigating ophthalmologists. By week 78, 145 out of the 242 (60%) patients who had active inflammation on enrollment were quiescent, 66% of whom were corticosteroid free and reduction of mean corticosteroid dose from 13.6 mg daily dose to 2.6 mg. Of those who were inactive at the start of the study 96 out of 129 (74%) were quiescent at week 78, with 93% of these patients steroid free.
The Sycamore study was a United Kingdom-based, investigator-initiated RCT that evaluated the efficacy of adalimumab in pediatric JIA patients with chronic uveitis despite the use of methotrexate (22). Early study results showed that adalimumab therapy was effective in controlling inflammation and had a much lower treatment failure rate compared to placebo triggering the prespecified stopping criteria. Adalimumab treatment demonstrated a 75% risk reduction (hazard ratio =0.25) compared to placebo in preventing uveitis flares in this population.
Golimumab (Simponi, Janssen Biotech, Horsham, PA) is a human monoclonal antibody against TNF-α that binds both the soluble and membrane forms of TNF. It was approved by the FDA in 2009 for the treatment of RA, psoriatic arthritis and ankylosing spondylitis and subsequently approved for ulcerative colitis. It is administered at a dose of 50 mg subcutaneously every month.
Although there has not been any RCTs evaluating the effectiveness and safety of golimumab in the treatment of NIU there have been several case reports and case series published in the past few years. A retrospective study looking at the effects of golimumab on 13 patients with refractory uveitis due to psoriatic arthritis, sarcoidosis, axial spondyloarthritis, JIA, Bechet’s disease and VKH previously on anti-TNF-α therapy showed ocular inflammation control in 12 out of the 13 enrolled patients (20).
Golimumab was also evaluated in 15 patients with refractory acute and chronic anterior uveitis and an underlying spondyloarthropathy (ankylosing spondylitis, psoriatic arthritis and non-radiographic axial SpA (23). These patients had active inflammation despite previous use of immunosuppression therapy and 10 out of 15 had also failed anti-TNF-α treatment (etanercept, infliximab or adalimumab). After a 23-month follow-up, 13 patients on golimumab achieved complete clinical remission. These findings were supported in the multicenter, prospective GO-EASY study in which the majority of the 93 patients with ankylosing spondylitis treated with golimumab for one year had a reduction in anterior uveitis occurrence rate and a significant improvement in disease activity (24).
Certolizumab pegol (Cimzia, UCB Inc., Smyrna, GA) is a pegylated anti-TNF-α antibody that is able to bind to both the soluble and membrane-bound forms of TNF-α. It has received FDA-approval for the treatment RA, Crohn’s disease, psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis and plaque psoriasis. It is administered by subcutaneous injection with a recommended initial dose of 400 mg given at weeks 0, 2 and 4. Subsequent maintenance doses of 200 or 400 mg are then administered every 4 weeks.
Similar to golimumab, there is limited data on the use of certolizumab in the treatment of NIU. A retrospective case series from 2014 evaluated the efficacy of certolizumab in 7 patients with active uveitis associated with Bechet’s disease, ankylosing spondylitis, Crohn’s disease, psoriatic arthritis and idiopathic retinal vasculitis. Ocular inflammation persisted despite use of other anti-TNF-α therapy. When given certolizumab, 5 out of 7 patients had achieved quiescence at mean follow-up of 10.4 months with no adverse events encountered (25).
The RAPID-axSpA trial was a multicenter, RCT with 325 patients assessing the efficacy and safety of certolizumab in patients with axial spondyloarthropathies (26,27). A post hoc report showed that the rate of uveitis flares at 24 weeks was found to be lower in the certolizumab group [3.0 (95% CI: 0.6–8.8) per 100 patient-years] than in the placebo group [10.3% (95% CI: 2.8–26.3) per 100 patient years]. Due to nature of the report, however, they were not able to perform statistical testing on the difference between the incidence of uveitis in the certolizumab group and control groups (26).
Etanercept (Enbrel, Immunex Corporation, Thousand Oaks, CA) is a recombinant fusion protein consisting of the extracellular ligand-binding portion of TNF-receptor 2 and the Fc portion of IgG1. It acts as a decoy receptor capable of binding to both the soluble and membrane bound forms of TNF-α. It is administered as a weekly subcutaneous injection at a dose of 50 mg. It is FDA-approved for the treatment of RA, ankylosing spondylitis, psoriatic arthritis, polyarticular JIA and plaque psoriasis.
Although early reports suggested promise in the use of etanercept in the treatment of NIU (28,29), results from other studies did not support these findings (30-32). An RCT investigating its use in the treatment of pediatric JIA-associated uveitis showed no difference in anterior segment inflammation between etanercept and placebo (32). In addition, in comparative studies etanercept was shown to be less effective than infliximab in managing inflammation (28,29).
There have paradoxical reports suggesting that etanercept may be a trigger for uveitis. A review of the reporting databases in 2007 found that there was a disproportionate amount of new uveitis associated with etanercept as opposed to the other TNF-α inhibitors (33), but it is difficult to assess whether this is due to causation, or simply related to a relative inability to prevent uveitis flares relative to other TNF blockers.
Rituximab (Rituxan, Genentech, Inc.) is a human-mouse chimeric monoclonal antibody that targets CD20, found on the surface of mature B lymphocytes. Rituximab was initially developed for the treatment of B cell lymphomas and was FDA-approved in 1997 for the treatment of Non-Hodgkin’s Lymphoma. Given the demonstrated importance of B cells in autoimmunity it was later studied and approved for use in the treatment of RA, granulomatosis with polyangiitis, microscopic polyangiitis, and pemphigus vulgaris.
There is growing evidence supporting the off-label use of rituximab in ocular inflammatory conditions including orbital inflammatory disease (34) and scleritis (35,36). A prospective trial evaluated rituximab therapy in 12 patients with refractory non-infectious scleritis on one or more systemic immunosuppression. At 24 weeks post-infusion, 9 of 12 patients (75%) met the primary outcome measures of inflammation reduction. Of note 7 of these 9 patients did require reinfusion to maintain inflammatory control (35).
With regards to the use of rituximab in the treatment of NIU, the evidence is limited to case reports and case series which have shown its effectiveness in cases with recalcitrant, sight-threatening uveitis (37-40). A retrospective case series looked at 11 patients (21 eyes) with refractory posterior uveitis treated with intravenous rituximab. Rituximab used alone or as a complimentary medication was associated with the resolution of ocular inflammation in 17 out of 21 (80.1%) eyes at 2 years follow-up (40).
Abatacept (Orencia, Bristol-Meyers Squibb Company), is a fully humanized fusion protein made of the extracellular portion of cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and the Fc fragment of IgG1. It is capable of binding to CD28 on the surface antigen presenting cells and therefore interfering with the interaction of co-stimulatory molecules with the T cell and inhibiting T cell activation. Abatacept is FDA-approved for treatment of RA, psoriatic arthritis and JIA (41).
Evidence supporting its use in NIU is limited to case reports and case series. The earliest report published in 2008 involves the use abatacept in a patient with refractory uveitis associated with psoriatic arthritis that did not resolve with the use of several agents including cyclosporine, methotrexate, rituximab and cyclophosphamide. Following the addition of abatacept to her regimen there was a significant improvement in her ocular inflammation (42). Following this report there were a number of publications highlighting its efficacy in the treatment of JIA-associated uveitis (43-46). A retrospective study conducted by members of the Multinational Interdisciplinary Working Group for Uveitis in Childhood (MIWGUC) evaluated the efficacy of abatacept in treating uveitis in 21 patients with JIA. They found that while 11 out of the 21 patients achieved initial inactivity, 8 of these patients had recurrence of their ocular inflammation; therefore only 3 of 21 patients achieved sustained disease quiescence (47).
IFNs are a class of naturally occurring signaling glycoproteins that have antiviral, antineoplastic, immunomodulatory and cytotoxic profiles. Type 1 interferons—IFN-α 2a, IFN-α 2b and IFN-β have been shown to be effective in treating various uveitic entities—specifically associated with Bechet’s disease (48-50) and multiple sclerosis (51).
A recent retrospective study evaluated the efficacy of IFN-α 2b therapy in 32 patients with refractory uveitis associated with Behcet’s disease who were not under control despite the use of corticosteroids and immunomodulatory therapy. Control of ocular inflammation was seen in 28 of 32 patients (88%) and the relapse rate decreased significantly from 1.68 relapses per patient per year to 0.11 relapses per patient per year in treated patients (49). A prospective study of 53 patients with active and vision-threatening Behcet’s uveitis that was refractory to corticosteroids and conventional immunosuppressive agents displayed the intermediate-term safety and effectiveness of IFN-α 2a therapy. At 1-year follow-up 45 of 53 patients (84.9%) experienced an improvement in their ocular inflammation and the flare rate was had decreased from 3.6 per year to 0.56, suggesting benefit of IFN therapy in refractory cases of Beh?et’s uveitis.
A prospective interventional case series evaluated the efficacy of IFN-α 2b on 12 patients with various non-infectious uveitic entities (52). These patients received IFN-α 2b subcutaneously at 3 million/IU/day for 3 days followed by an increase to 6 million/IU/day for 2–4 weeks and then had dose adjustments depending on their response. In this study 83% of patients had a positive clinical response to treatment. IFN-α 2a has also been shown to be effective in the treatment of Behcet’s disease and other uveitic entities either as a stand-alone therapy or in combination with other immunomodulatory agents (50,53).
Recent evidence has supported the use of systemic IFN therapy in the treatment of refractory uveitic macular edema (54-58). A retrospective study showed that both non-pegylated and pegylated IFN-α 2a were both similarly effective and well tolerated—with the pegylated form offering the advantage of longer duration of activity and therefore less frequent dosing (58). Another retrospective study evaluating pegylated IFN-α 2a in 7 patients with refractory uveitic macular edema also showed improvement in the central macular thickness and visual acuities of these patients (56).
Interleukin-1 inhibitors such as Anakinra (Kineret, Swedish Orphan Biovitrum, Stockholm, Sweden) have been FDA approved for the treatment of RA but there is no current evidence for their efficacy in the treatment of NIU. A trial of the drug gevokizumab showed initial suggestion of benefit, but the study and drug were withdrawn after subsequent unsuccessful studies (59). There is ongoing recruitment for an RCT looking at comparing the efficacy of anakinra to that of adalimumab and tocilizumab for the treatment of NIU.
IL-6 is a pleiotropic cytokine that is upregulated during inflammatory and infectious processes. It is involved in the differentiation of CD4 cells into Th17 cells—a subgroup that has been linked to several immune-mediated diseases. Elevations in IL-6 have been found in the ocular fluid of patients with refractory and chronic uveitic conditions including VKH, Behcet’s disease and sarcoidosis (60-62).
Tocilizumab (Actemra, Genentech Inc.), a monoclonal antibody, was the first biologic drug targeting interleukin-6 receptors (IL-6R) to demonstrate effectiveness in humans. Tocilizumab binds to both membrane-bound and soluble IL-6Rs. It has been FDA approved for RA, poly-articular and systemic JIA, and giant cell arteritis.
Several case reports and case series have demonstrated consistent efficacy of tocilizumab in treating uveitis-induced macular edema (63-66). The STOP-Uveitis trial, a phase II, multi-center, RCT with 37 NIU patients evaluated the safety efficacy of tocilizumab at two doses: 4 and 8 mg/kg (67). This small randomized trial showed improvement in visual acuity, central macular thickness and vitreous haze scores in the two groups the study during a relatively short follow-up period of 6 months. The APTITUDE trial is an ongoing phase II clinical trial that is evaluating tocilizumab as a therapeutic option in anti-TNF refractory JIA-associated uveitis (68).
The Janus kinase (JAK) family of enzymes are involved in the downstream signal of various cytokine receptors that are involved in controlling cell growth and the immune response including IL-2 and IL-6 (69,70). These enzymes act to phosphorylate STAT transcription factors which have an effect on gene transcription. Drugs inhibiting their activity block will therefore interrupt cytokine signaling. Several JAK inhibitors have been FDA approved for the treatment of RA, psoriatic arthritis and ulcerative colitis (71-73).
Tofacitinib (Pfizer, Inc., New York City, NY) is a JAK inhibitor with selectivity for JAK1 and JAK3. There are no current studies that have shown its effectiveness in the treatment of NIU, however several case reports have shown possible synergistic effects when used with other immunomodulatory agent such as methotrexate (74,75). The safety and efficacy of Filgotinib, a JAK1 inhibitor, is currently being evaluated in a multicenter RCT for the treatment of uveitis.
There is mounting evidence compiled over the last two decades supporting the use of biologics as a safe and effective therapeutic option in the management of NIU, with special emphasis on the TNF blockers. Of the five approved TNF blockers, the four monoclonal antibodies (adalimumab, certolizumab, golimumab, and infliximab) have all demonstrated benefit for the treatment of NIU in variably sized clinical reports and case series. The fusion protein etanercept, in contradistinction, has been demonstrated to not be beneficial for the treatment of NIU. The VISUAL trials led to the FDA-approval of adalimumab in adults with NIU in 2016 and subsequently, the publication of the SYCAMORE study led to approval for the treatment of NIU patients over the age of 2 in 2018. Although TNF blockers are clearly very effective in many cases, patients must be carefully monitored for potential side effects, most specifically infection and demyelinating disease. Regarding infection, tuberculosis and hepatitis B and C must be ruled out prior to starting a TNF blocker, and a high level of vigilance for incident infections must be maintained. Demyelination should be considered prior to starting TNF blockers in any NIU patient, especially patients with intermediate uveitis, due to the known association with multiple sclerosis in some cases. Additionally, TNF blockers are relatively contraindicated in patients with congestive heart failure or prior malignancy. Contemporaneously to the advent of TNF blockers, the last decade has seen an exponential increase in novel biologics approved for inflammatory diseases related to uveitis, and many of them have been reported on in the treatment of NIU with promising results. Ongoing study is needed to further define the role of biologic drugs in the treatment of NIU in the future.