Abstract: The most prominent causes of loss of vision in individuals over 50 years include age-related macular degeneration (AMD), glaucoma, and diabetic retinopathy (DR). While it is important to screen for these diseases effectively, current eye care is not properly doing so for much of the population, resulting in unfortunate visual disability and high costs for patients. Innovative functional testing can be unified with other screening methods for a more robust and safer screening and prediction of disease. The goal in the creation of functional testing modalities is to develop highly sensitive screening tests that are easy to use, accessible to all users, and inexpensive. The tests herein are deployed on an iPad with easily understood and intuitive instructions for rapid, streamlined, and automatic administration. These testing modalities could become highly sensitive screenings for early detection of potentially blinding diseases. The applications from our collaborators at AMA Optics include a cone photostress recovery test for detection of AMD and diabetic macular edema (DME), brightness balance perception for optic nerve dysfunction and especially glaucoma, color vision testing which is a broad screening tool, and visual acuity test. Machine learning with the combined structural and functional data will optimize identification of disease and prediction of outcomes. Here, we review and assess various tests of visual function that are easily administered on a tablet for screening in primary care. These user-friendly and simple screening tests allow patients to be identified in the early stages of disease for referral to specialists, proper assessment and treatment.
Age-related macular degeneration (AMD), glaucoma, and diabetic retinopathy (DR) are the most prominent causes of vision loss in those older than 50 years in the developed world. Screening for each of these diseases is crucial, and eye care currently fails a large segment of our population, with catastrophic consequences in visual disability and cost. Here, we evaluate and review 4 visual function tests that are administered on a tablet for screening in primary care. These tests would also be ideal for combined use with any other screening modalities such as non-mydriatic fundus photography with automated cameras. They can also be easily incorporated into eye clinics for research on these same diseases. In addition to reviewing published results, we also describe in more detail the results of a study with one test, the cone photostress recovery time (PRT) for detection of DME.
PRT is a classic marker for macular disease, such as AMD and DME associated with DR (1,2). After bleaching the photoreceptor photopigment, PRT is the time to recover visual sensitivity (regeneration of photopigment) (3). While of academic interest, this measure of visual function has not become a part of routine evaluation of the macula that is dominated by optical coherence tomography (OCT). However, in the recent paradigm shift to wider screening in primary care settings, and because PRT is affected early in disease (4), a novel, inexpensive photostress recovery test, the Annulus Adaptometer, can screen subjects for early referral.
Other measures of visual function such as brightness-perception, which is a subjective test, and the visual evoked potential (VEP), which is an objective test, provide key information for evaluating the health of the macula and visual pathways. Brightness sense or VEP imbalance between the eyes is a sensitive but nonspecific marker for visual dysfunction. While VEP is unsuitable for primary care, many psychophysical tests of brightness imbalance have been described (relative color saturation (5), Pulfrich effect (6), non-rivalrous brightness sense (7), and rivalrous brightness sense (8), that have been useful for studying a variety of diseases, including macular disease (6,9,10), optic nerve diseases (7,8,11,12), (including glaucoma) (13,14), and amblyopia (6,9,15-17). However, only the brightness sense test is suitable for primary care and telemedicine. Glaucoma in particular is well-poised to be assessed by brightness-perception as brightness asymmetry is very common in glaucoma and is significantly correlated with visual field loss (14).
Color Vision defects in glaucoma are documented in literature (18-22). The common age-related eye diseases of interest, including glaucoma, AMD and DR produce blue-yellow color vision deficiencies, at least in the pre-clinical or early stages (22-24).
Uncorrected refractive error (URE) causes visual disability worldwide (25), and in the underserved in metropolitan areas (Carol Horwitz, Director, Institute for Health Equity Research, personal communication). Deep learning (DL) can measure spherical error (only) from retinal photos (26), but not full refraction. Further, high technology, such as an autorefractor is not practical for screening. Therefore, a practical solution would be a simple app to identify URE for optometric referral, and uncorrectable decreased acuity for ophthalmic referral, a broad safety net for otherwise undetected eye disease.
Such testing modalities could become highly sensitive screenings for early detection of potentially blinding diseases.
Due to the fact that retinal functional testing is a broad topic, we did not perform a formal PubMed search, but rather consulted experts in the field of Ophthalmology for references on functional testing modalities for various disorders. These include all known handheld and portable devices suitable for telemedicine. We then reviewed all of those references for possible connections and applications in a primary care clinic. From all such tests, we selected those which fell into the 4 large categories described in the Introduction that have been the most widely researched and have the broadest potential applicability. These are PRT, brightness balance, color vision, and visual acuity. Visual acuity has the evident capacity to detect eye disease in the screening programs to which all these measures are directed, it suffices to demonstrate the simplicity and ease of testing on a portable device, we evaluated the benefits and limitations, while assessing the reproducibility, specificity, and sensitivity for any test. Herein, we review, demonstrate, and evaluate 4 new tests of visual function that are easily administered on a tablet for screening in primary care. We compare these selected high impact functional tests with other devices in the literature. In order to avoid bias towards the testing portfolio on the iPad, we clarify that the goal of the review is to identify the tests’ advantages and application in primary care, and thus only briefly discuss a large number of prior techniques that fail this criterion.
Four new functional tests, each deployed on a handheld device, are described here in comparison to tests in the literature in each of the four main categories just mentioned. The new tests comprise the iOS DiagnosticGame?, and are deployed on an iPad (iPad mini-4, Apple Inc, Cupertino, CA) with easily understood and intuitive “Touch the Screen” instructions for rapid (~30 sec per eye), streamlined, and automatic administration. The panel of applications includes one in each category: a Cone Photostress test (the Annulus Adaptometer, sensitive to central macular dysfunction), brightness balance perception, color vision testing, and visual acuity check.
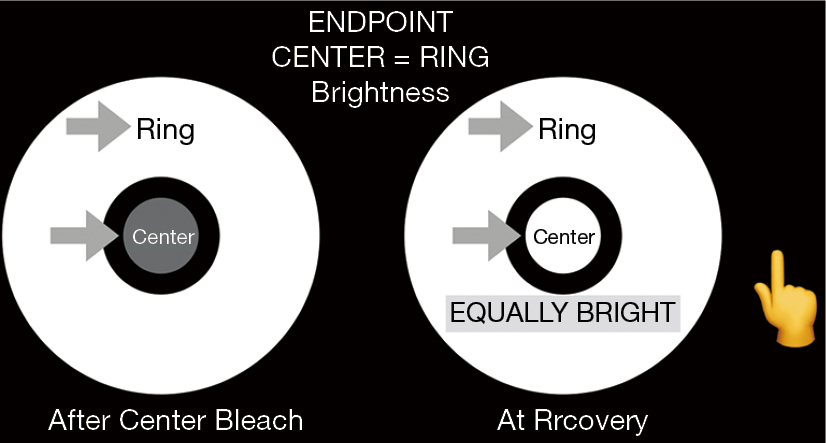
For the photostress recovery test, called the Annulus Adaptometer, the iPad screen luminance is adjusted to 330 cd/m2. This was established as a dependable calibration endpoint by measuring the central luminance of the screens of 5 iPads with an International Light photometer: the range was 331–351 cd/m2, with test/retest variation 1%. The non-dilated eye is then tested with near correction at 16” and fellow eye occluded. The Annulus Adaptometer test takes about one minute per eye. The central 2 degrees of the macula are bleached for 30 seconds by central target fixation at the fixed brightness of the device screen (Figure 1).This is compared in brightness, after bleaching, to an annulus of surrounding unbleached retina of the same eye to measure the cone photostress recovery time (CPRT). The recovery time is the time for the dim appearing central bleached circle to appear of equal brightness to the surrounding annulus of unbleached retina, a relative endpoint that is easily discernible. Hence, the eye serves as its own control. Further, bleaching for 30 secs generates a sufficiently dark after image for comparison, much less than the time for peripheral rod spot bleaching (4).
CPRT was measured in a group of 37 diabetics who also underwent spectral domain OCT (SD-OCT) measurement of central foveal thickness (CFT) at the NYU Medical Center diabetes outpatient clinic. DME was defined as CFT >300 μm. The CPRT was then correlated with presence or absence of DME (15-17).
Brightness perception by the two eyes is precisely balanced in humans, and the Amblyometer? of the iOS DiagnosticGame?, detects when brightness is imbalanced (Figure 2). The test is based on the principle of the Wheatstone bridge, an electrical circuit used to measure an unknown electrical resistance with high precision by balancing two legs of a bridge circuit, and is the first such vision test (https://patents.google.com/patent/US9560960B2/en). Reduced brightness in one eye causes conduction delay of nerve impulses from that eye to the brain, which is recorded as a prolonged latency in the VEP (27). In glaucoma, visual fields assess focal damage, which is summated by the mean deviation (MD) index for comparison to global nerve loss measured by VEP or brightness sense imbalance.
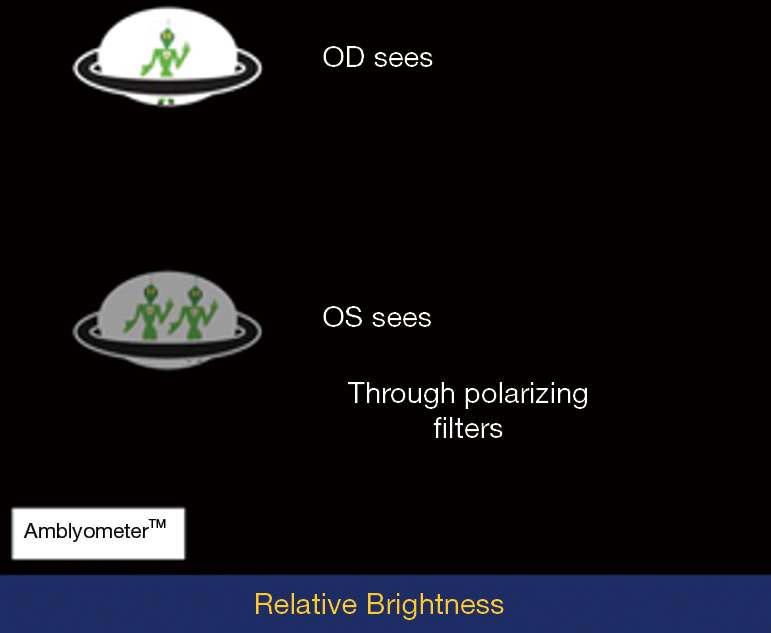
The subject wears polarizing glasses of opposite polarity on the right and left eyes, and observes test objects that are also polarized to dissociate the perceptions of the two eyes under binocular viewing. The subject then touches the brighter object, while the program titrates individual brightness with staircase algorithms, aka ascending and descending methods of limits (13). The mean and variance of 4 repetitions are calculated, and high variance requires repeat testing. The mean testing time (4 tests) is 2 mins. The difference between the two eyes, if any, is measured in steps of 0.3 log units of stimulus brightness.
This game was tested on 286 school children for accuracy in detecting amblyopia.
The automated NeuroColor? game of the DiagnosticGame? app was designed to detect hereditary and acquired color vision (CV) defects. It presents copies of the AO Hardy-Rand-Rittler (HRR) pseudoisochromatic color plates on the iPad so that only touching the color is required.
In this game, the color plates are: (I) a demonstration plate visible to all except those malingering or totally blind, (II) a control plate with no color symbols, (III) 2 plates for detecting red-green defects and (IV) 2 plates for detecting blue-yellow defects (Figure 3). If a plate is missed, it is presented once more. This game was tested on 211 children for accuracy in measuring color vision in comparison to the gold standard HRR color plates.

The visual acuity test, the digital tumbling E visual acuity (TEVA) game of the DiagnosticGame? app, is based on matching vertically aligned Es (or Cs), and is independent of literacy (Figure 4). The best vision protocol includes testing with a +2.5 D lens over the distance glasses, and, for visual acuity less than 20/40, the addition of a pinhole disc simulating universal focus. The accuracy of this testing is reliant on the appropriate distance between the iPad and the patient’s eyes, and therefore proper distance is needed for accurate results. The possible abnormal outcomes and proposed dispositions are: uncorrected refractive error (URE) for optometric referral, and uncorrectable decreased acuity for ophthalmic referral. This Digital TEVA at 16 inches was tested on 65 children.
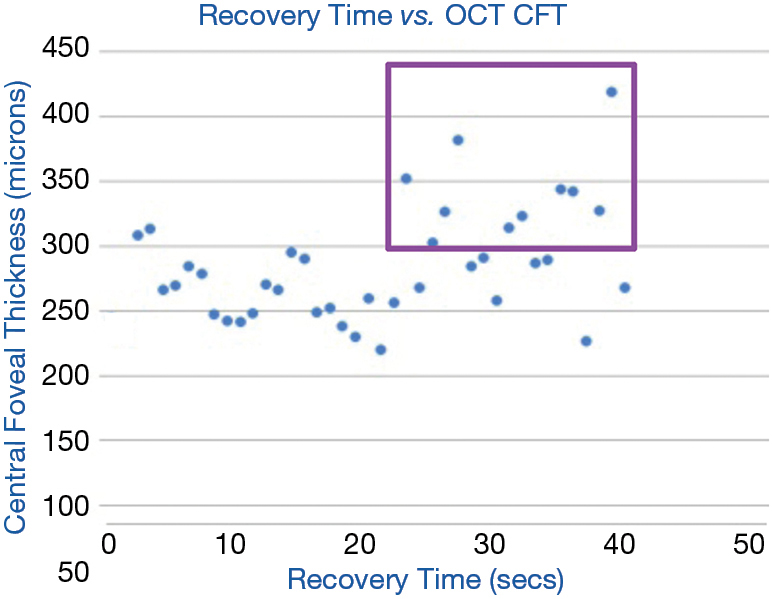
The CPRT in 37 diabetics was associated with the severity of DME (28): recovery time greater than 21 seconds correlated significantly with central foveal thickness (CFT) >300 μm as measured by OCT (P=0.02, Chi square). True positives are points included in the purple box (Figure 5). As a test for DME, sensitivity was 83% and specificity was 55%.
The Amblyometer? achieved a sensitivity and specificity of 100% for identifying amblyopic children in an office practice (16). It was further validated through screening 208 school children, with highly correlated test and retest scores (r2=0.985, P<0.00001) (16), and the accuracy for amblyopia detection was as follows: Of the 208 children that were recruited as subjects and tested at school, 121 were girls and 87 were boys with ages from 3 to 14 years and a mean of 7.8 years. In screening these children, 2 amblyopes were detected (2 true positives) and 206 true negatives (17).
The NeuroColor? game was validated in school children, (American Academy of Pediatrics, 2019) (17) in comparison with the AO HRR plates. 211 children were tested, 205 children tested were normal, and 6 children displayed a color vision deficiency with both the AO HRR color plates and the NeuroColor? game, identical results. Further, of the 6 abnormal results, 5 were bilaterally identical, classified as hereditary CV deficiency by both tests. The 1 acquired defect was monocular and occurred in the amblyopic eye of a 10-year-old. The mean testing time was 53 seconds.
Digital tumbling E visual acuity (TEVA) at 16 inches was validated (17) by comparison to Snellen acuity with letter sizes of the same visual angles on 65 children. The results were significant correlated and no significant differences were found. The mean testing time for both eyes was 89 seconds. The mean difference between iPad and E-Chart visual acuities with pinhole was 0.02 logMAR, with 95% limits of agreement from ?0.08 to +0.11 logMAR.
A strength of functional tests for screening is that they are generally not specific for one disease, such as Color fundus photography (CFP) specificity for AMD or DR, but rather are sensitive to many vision disorders that merit specialist referral. This low specificity and high sensitivity is key to effectively screening, rather than diagnosing, eye diseases. However, a desirable specificity still applies, in that a positive test has a high probability of identifying a true positive that is meaningful in population screening, i.e., a significant vision disorder. Further, they provide strong protection against false negatives from other tests, such as from AI, that could “pass” on serious disease.
A major test advantage and innovation of the CPRT on the iPad is that it is relative: a bleached center is compared to the unbleached surround. Further, the test is quick, about a minute per eye, and is sensitive, e.g., to DME (28) (Figure 5). The abnormally increased recovery times in the diabetic subjects with normal CFT may be due to other DR pathology to be determined. Thus, the lower specificity is not necessarily a disadvantage here, and in fact may be useful in research and eventually clinical care. For example, prolonged recovery times may be due to macular ischemia in the absence of edema.
Research has shown that PRTs are delayed with aging, AMD, diabetic retinopathy, and central serous retinopathy. Repeatability was excellent in some, e.g., Newsome et al. (29), but not others (30,31). The existing tests are high quality, but still require specialized equipment, expert personnel, and therefore remain impractical, especially in a primary care setting. For example, the method of Newsome is considered excellent, and yet is not suitable for the reasons given. Thus, although good repeatability can be achieved in modern research settings (4,32), there is currently only one reliable photostress test proposed for detecting disease economically and easily, the cone photostress test (CPRT) on the iPad. It is quite analogous to the Newsome test, also with good repeatability, and thus just as effective in screening. However, in contrast to the Newsome test, the CPRT on the iPad, a widely available, inexpensive commercial device, provides eminent practicality. In the CPRT, the central bleaching and measured recovery in brightness to surrounding unbleached retina is impressively all done on the iPad, providing complete ease for patients.
Many devices used varying methods of bleaching and endpoints. Technical expertise and cost also vary (28,30,31,33). With the AdaptDx dark adaptometer (4), delayed rod recovery time, beyond 6.5 minutes, was a 91% sensitive and specific early marker for AMD (4). However, the adaptation time reported of cone recovery in a central photostress test was also significantly prolonged in most (69%) AMD eyes (34). Required testing was much shorter than with the AdaptDx. Normal recovery was complete in 20 to 50 seconds, and longer in most AMD subjects. Further research comparing rod dark adaptation (DA) and cone PRT found both had excellent diagnostic capacity for early AMD (32), suggesting cone testing is rapid and effective in screening. In addition, the Annulus Adaptometer has a more comfortable luminance (~330 cd/m2) than other photostress tests (34).
In glaucoma, visual fields assess focal damage and global nerve loss is measured by VEP, which is summated by the MD (mean deviation) index for comparison to or brightness-sense imbalance. In glaucoma patients, the MD index significantly correlated with prolonged VEP latency (35) and similarly for the pattern reversal VEP.(11) More important for screening, brightness-sense imbalance in a validated office test analogous to the Amblyometer? of the iOS DiagnosticGame?, was found in 100% of 20 primary open angle glaucoma (POAG) patients and in only 3 of 61 age-matched controls (13); in another study of POAG patients, 86% of 28 (14) patients had brightness imbalance significantly correlated with visual field loss. The Amblyometer? of the iOS DiagnosticGame?, which detects when brightness is imbalanced, is analogous to measuring an unknown electrical resistance with high precision by balancing two legs of a Wheatstone Bridge circuit, the first vision test of its kind. The high sensitivity of this tool can capture glaucoma patients and others that need referral.
Color vision screening is of particular importance because color vision defects in glaucoma are documented in literature (18-22). B/Y defects in particular increase with age, and with the presence of cataract. Our common age-related eye diseases of interest (glaucoma, AMD and DR) all produce blue-yellow color vision deficiencies, at least in the pre-clinical or early stages (22-24), and therefore they are non-specific. However, only 20% of those younger than 75 years of age, patients with cataracts included, made errors on the Adams desaturated D-15 (36) but B/Y defects were 30-50% in ages greater than 75, which was mostly due to aging and cataract. This suggests that subjects younger than 75 with B/Y defects, but not older, should be referred for evaluation. The NeuroColor? game has been validated, and will serve these functions easily, as has just been shown. This compares favorably to complex tests of hue discrimination and allows quick, effective testing on a handheld device equivalent to standard isochromatic plate testing.
The digital tumbling E visual acuity game for testing in the office or for screening is easily accessible to assess patients’ visual status. Individuals are asked to identify shapes without the need to read letters, and therefore literacy is not required for (Figure 4). The possible abnormal outcomes and proposed dispositions are widely applicable to general populations: uncorrected refractive error (URE) for optometric referral, and uncorrectable decreased acuity for ophthalmic referral
With telehealth programs currently expanding rapidly in the face of COVID-19, and clearly here to stay, these accessible, user-friendly, cheap, and simple screening methods will be an ideal part of this future. These screening tests allow patients to be identified in the early stages of disease for referral to specialists, proper assessment and treatment.