Abstract: Dramatic advances in retinal imaging technology over the last two decades have significantly improved our understanding of the natural history and pathophysiology of non-neovascular age-related macular degeneration (AMD). Currently, aside from micronutrient supplements, there are no proven treatments for non-neovascular or dry AMD. Recently, a number of pharmacological agents have been evaluated or are under evaluation for treatment of patients with end-stage dry AMD manifesting as geographic atrophy (GA). It may preferable, however, to intervene earlier in the disease before the development of irreversible loss of visual function. Earlier intervention would require a more precise understanding of biomarkers which may increase the risk of progression from early and intermediate stages to the late stage of the disease. The development of optical coherence tomography angiography (OCTA) has allowed the layers of the retinal microcirculation and choriocapillaris (CC) to be visualized and quantified. Flow deficits in the CC have been observed to increase with age, particularly centrally, and these flow deficits appear to worsen with development and progression of AMD. As such, OCTA-based CC assessment appears to be a valuable new biomarker in our assessment and risk-stratification of AMD. Alterations in the CC may also provide new insights into the pathophysiology of the disease. Enhancement of choriocapillaris function may also prove to be a target of future therapeutic strategies or as a biomarker to monitor the effectiveness of therapy. As such, CC imaging may be anticipated to be an integral tool in the management of dry AMD.
Age-related macular degeneration (AMD) is the most important cause of severe vision loss among the elderly in developed nations. As life expectancy continues to increase, the prevalence of this disease is expected to rise dramatically over the next few decades and AMD will become an increasingly important major health problem worldwide with global socio-economic implications (1-7). The majority of patients with AMD manifest the non-neovascular form of the disease, accounting for 85–90% of all cases of AMD (8,9). The precise natural history of non-neovascular AMD appears unpredictable for a given eye, with some eyes developing macular neovascularization (MNV) and others progressing directly to geographic atrophy (GA) during the late stages of the disease (10). It is important to note that atrophy is not exclusive to dry AMD, as it can precede or follow MNV, and some suggested using the term macular atrophy (MA) to describe atrophy in eyes with neovascular AMD (11). While effective anti-vascular endothelial growth factor (VEGF) therapeutics are available to treat the neovascular form of AMD, no proven therapy is currently available for the prevention or treatment of atrophy (12). The Age-related Eye Disease Study (AREDS) trial demonstrated that micronutrient antioxidant supplements could reduce progression of intermediate AMD to late AMD, the benefit was primarily in reducing the development of MNV, with no clear benefit on preventing central atrophy (13-16). Recently, lampalizumab (17), a selective complement factor D inhibitor, failed to show any benefit in reducing the enlargement of GA lesions in the Chroma and Spectri Phase 3 trials (18). These studies did enroll patients with high risk for GA progression, and showed that in these eyes the GA lesions enlarged at a rate of approximately 2 mm2 per year. More recently, Liao et al. (19) published the results of the Phase 2 Filly study pegcetacoplan, an inhibitor of complement factor 3 activation (APL-2), as a potential therapy for GA. There appeared to be a reduction in the GA enlargement rate compared to sham treatment over 12 months, though there was a higher incidence of exudation in the treated patients. Phase 3 trials of pegcetacoplan (19) and avacincaptad pegol (20) are currently in progress. A number of other therapeutic agents are also currently under investigation, but even if these are successful, it is likely they would only slow, but not stop or reverse the progression. Thus, there has been an increasing interest in earlier intervention prior to the development of atrophy. Early intervention trials, however, require identifying subjects who are at the highest risk of progression, in order to effectively design a trial that is of practical size and duration to be clinically feasible. Thus, there has been a strong focus recently on identifying imaging biomarkers which could be used for risk stratification and prognostication. A number of structural OCT biomarkers including high central drusen volume, subretinal drusenoid deposits, hyporeflective drusen cores, and intraretinal hyperreflective foci have been defined (21,22). In addition, to the photoreceptor and retinal pigment epithelium (RPE) that can be studied by structural OCT, the inner choroidal vasculature, and in particular the choriocapillaris (CC) is thought to be important in the pathophysiology of AMD. The development and continued evolution of optical coherence tomography angiography (OCTA) technology, has now allowed the three dimensional retinal microvasculature and CC to be visualized and quantified in a non-invasive manner. Thus, OCTA has provided a key technological tool to more precisely explore the role of the choroidal vasculature in the pathophysiology of AMD (23,24).
In this review, we describe the current studies of the CC in eyes with dry AMD evaluated using OCTA, describing the role of CC flow deficit as a risk factor that could be prognostic for the progression of the disease to late stages. We begin by reviewing the classification of non-neovascular AMD, including the hallmarks of the disease and the current retina imaging technologies for identifying and monitoring its progression. Subsequently, we will summarize the main quantitative CC studies conducted in eyes with early, intermediate and late AMD using OCTA. Finally, we discuss the impact of the OCTA CC findings as prognostic factors in the progression of the disease and the current limitations for the use of OCTA.
Histopathological and OCT-based studies have reported that the earliest changes in the natural history of non-neovascular AMD occur at the level of the interface between retina and choroid, involving the outer segments of the photoreceptors, retinal pigment epithelium (RPE), Bruch’s membrane (BM) and CC (25-29). Early changes in dry AMD include the deposition of material within the BM and between the BM and the RPE, causing thickening in the sub-RPE space with deposition of soft drusen, subretinal drusenoid deposits (SDD) and pigmentary abnormalities associated with attenuation, discontinuity and disruption of the RPE (30-32). Subretinal drusenoid deposits has been recognized as a distinctive phenotype in eyes with non-neovascular AMD in which the subretinal deposits are located internally to the RPE in contrast to the soft drusen, located externally to the RPE (33).
From these early changes, the dry form of AMD can progress to either MNV and/or atrophy. In the atrophic process the RPE degeneration is associated with photoreceptor degeneration and CC attenuation (34,35).
Currently, several AMD classification schemes and grading systems have been proposed in the effort to assess the severity of the disease and eventually guide physicians and researchers in both diagnosis and management of AMD (36-40). Most of these have been based on standard color fundus photographs which provide an easy translation to clinical examination and ophthalmoscopy. The most recent such classification system was proposed by the Beckman Initiative for Macular Research (40). This classification is based on lesions assessed within 2 disc diameters of the fovea in subjects older than 55 years, using either color photos or clinical exam. Eyes with no visible drusen or pigmentary abnormalities are considered to be normal or have no evidence of AMD. Small isolated drusen, also termed drupelets (<63 μm) are considered physiologic aging changes and not considered as part of the AMD spectrum, potentially with no increased risk of late AMD compared to the general population. Medium drusen (ranged 63–125 μm) in the absence of pigmentary abnormalities are considered as evidence of “early” AMD, while isolated large drusen or large drusen associated with pigmentary changes are hallmarks of “intermediate” AMD. The term “late AMD” implies the presence of MNV or GA, regardless of whether the foveal center is involved or not.
In 2017, the Classification of Atrophy Meeting (CAM) group recommended a multi-modal retinal imaging approach to optimally assess atrophy (41), proposing optical coherence tomography (OCT) as the base or reference technology. The CAM group also developed a consensus nomenclature and a set of OCT-based definitions for atrophy associated with AMD (42). The term “complete RPE and outer retina atrophy” (c RORA) was introduced, and defined by the presence of choroidal hypertransmission and attenuation of the RPE band ≥250 microns with overlying photoreceptor loss. More recently the CAM group also introduced the term incomplete retinal pigment epithelial and outer retina atrophy (iRORA) to describe an intervening phase in the transition from intermediate AMD to cRORA (43). The detection of early atrophic changes in eyes with intermediate AMD may be particularly informative and useful for clinical trials aiming to develop new therapeutic options for dry AMD.
The precise interaction of the photoreceptors, RPE, and CC in the pathophysiology are incompletely understood (35). There is also uncertainty as to the precise transition between normal aging and early AMD. The Alabama Study on Early Macular Degeneration (ALSTAR2) proposed the hypothesis that early AMD is a disease of micronutrient deficiency and vascular insufficiency, due to detectable structural changes in the retinoid re-supply mechanism from the CC to the photoreceptors, causing dysfunction of the rod-photoreceptors (44). This study confirms the need for better understanding of the early AMD changes, including the CC, in order to develop useful preventive strategies for dry AMD.
Although color photographs have been the historical gold standard for classifying AMD, most clinicians and researchers have now moved to a multimodal imaging approach (24), including infrared reflectance, autofluorescence, and OCT for the monitoring of AMD. These technologies, however, do not allow the CC to be evaluated in detail.
OCTA employs motion contrast to detect blood flow and acquires three-dimensional volumetric information of the retina and choroid to provide high-resolution, depth-resolved segmentation of the vascular layers, including the CC. Given the vascular images of OCTA are co-registered with structural OCT B-scans data, it is possible to correlate the vascular changes with structural features highlighted on the OCT B-scans. OCTA may be performed using both spectral-domain (SD) and swept-source (SS) devices. SD-OCTA is characterized by a broad bandwidth light source which is coupled with a spectrometer, while SS-OCTA is equipped with photodetectors and a tunable laser light source that operates through a range of frequencies. Moreover, SS-OCTA is characterized by a generally faster rate of acquisition of the images, at 100,000–400,000 A-scans per second, versus in the range of 70,000 A-scans per second for most commonly available SD-OCTA systems. The speed of acquisition is particularly important because OCTA relies on decorrelation between sequentially acquired OCT B-scans, therefore the quality of the resultant image depends on the velocity of acquisition. In addition, SS-OCTA operates at a wavelength of ~1,050 versus 840 nm for SD-OCTA, allowing a deeper penetration of the signal through the RPE, pigment deposits and drusen, thereby providing better visualization of the choroid and CC and a more detailed high-resolution high-definition image (45). The improved imaging speed and deeper penetration has significantly improved our capacity to visualize and quantify the CC in the setting of disease. Typical CC en face OCTA images are characterized by a granular appearance, in which small dark regions, are interspersed with bright areas, indicating the presence of flow. The dark regions are believed to represent areas with blood flow below the decorrelation threshold, making the blood flow not detectable on the en face OCTA image. These areas can either represent healthy areas with slow flow or areas with absent flow secondary to either normal intercapillary spacing for age or a pathological process involving the CC (23). Our group has reported that an increase in CC FD may be found in healthy eyes as result of the physiological aging process, and that this increase is most pronounced centrally (46). The dark areas related to an impairment of the CC are called “flow deficits” (FD), and also referred to by some investigator as signal voids (23,47-51). The size of these flow deficits may determine whether they are pathologic or not, and some authors have suggested that the normal intercapillary distance (ICD) should be taken into account and small physiologic FDs should be excluded in quantitative studies of the CC (52). Histological studies (53) have shown that the normal ICD diameters vary from the central macula to the periphery and that the intercapillary spaces in the posterior pole are distributed in a dense capillary meshwork pattern with diameters ranging from 2 to 20 microns. This pattern has been confirmed by in vivo SS-OCTA (51,54) and Zhang et al. (52) have proposed to exclude spaces smaller than 24 μm in diameter from the CC FD quantification.
Given that the CC appears to be progressively impaired with age, and because of interest in precisely studying the CC in AMD, quantification of the CC has a topic of great interest (46,49,50,55). Thus far there is no universally accepted consensus protocol to quantify CC FDs, but there has been steady progress in understanding the pitfalls and limitations of various approaches. In 2017, Al-Sheikh et al. proposed the Otsu’s global thresholding methodology to visulalize the CC FDs (48). The Otsu’s method reports the FDs following a bimodal distribution on grey-level histograms (56). Other researchers used the mean pixel value in the outer retina layer (ORL) as a global threshold to quantify the CC FDs (23,57,58). This methodology makes the assumption that the ORL shares the same systemic noise characteristics as the CC, without considering that the CC is located under the RPE complex, which can result in a rise in the noise level due the scattering nature of the RPE. Carnevali et al. evaluated CC FDs using the mean pixel value of the CC as a global threshold (59). The multiscale Hessian enhancement was also proposed as a morphometric methodology to measure CC in SS-OCTA (60). At present, the most commonly used methodology is to binarize, detect and quantify CC FDs with Phansalkar’s method (61), which is a local thresholding methodology (46,47,49,50,62-66). This methodology defines the CC FDs in all the areas is which flow is lower than the given Phansalkar radius set as a threshold. A limitation of this method is that the selection of the Phansalkar radius has to take into consideration the actual size of the pixels in the image. Recently, the fuzzy C-means (FCM) self-clustering algorithm has been proposed to segment the CC FDs (66,67). This methodology automatically assigns all pixels in a CC scan into clusters based on histogram distribution. Another quantification algorithm, the standard deviation (SD) method, takes into consideration the mean and SD from the reference normal database to determine a global threshold. Pixels with an intensity lower than one SD below the reference mean were considered as FDs. To summarize, the major limitation in assessing the FDs is the lack of a globally approved and validated approach to analyze the CC.
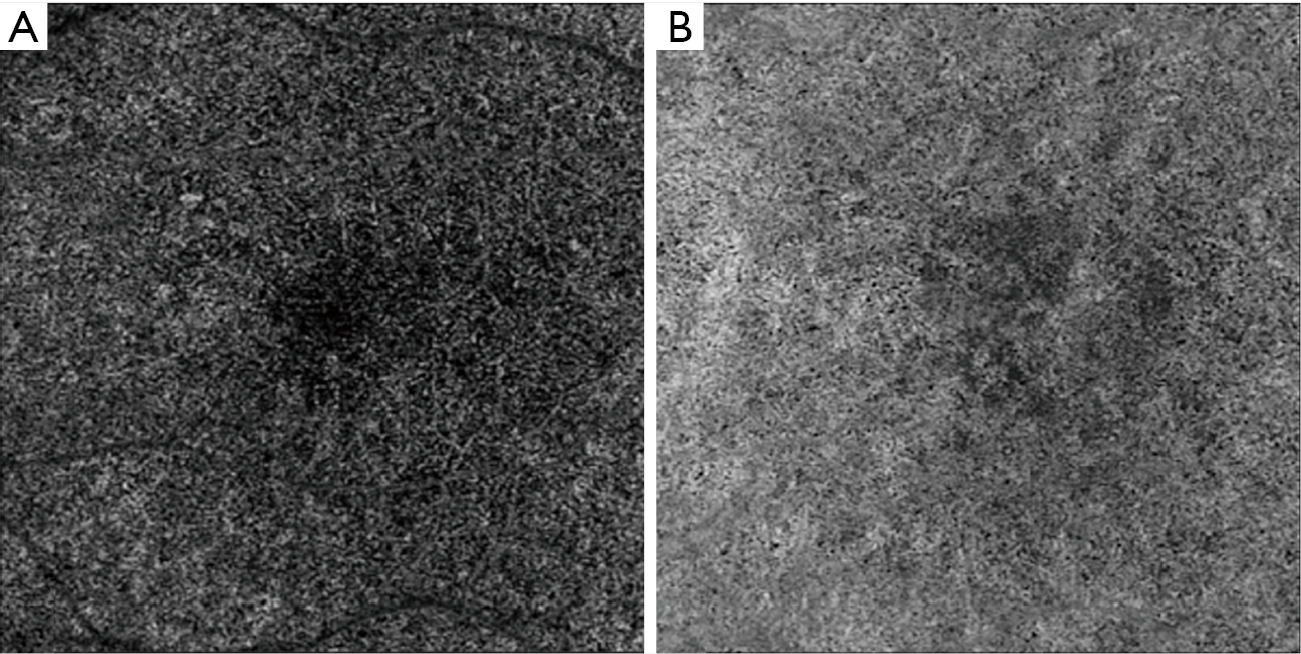
Early and intermediate AMD are characterized by drusen and/or pigmentary abnormalities on OCT B-scans and OCTA has allowed the correlation between in vivo microvascular alterations in the CC to structural changes in the retina and RPE (55,68), CC en face OCTA images of early non-neovascular AMD eyes showed a general increase in choriocapillaris FD when compared to age-matched healthy controls (69). This suggested that the transition from normal aging to early AMD may be reflected in the CC. Our group has investigated CC features in eyes with intermediate AMD using both SD- and SS-OCTA (70,71). We observed an increase in the CC flow deficits size, but no overall change in CC FD% in intermediate AMD eyes compared to healthy controls, though the regions directly below drusen could not be studied as this study utilized SD-OCTA. Shadowing artifacts due to drusen are an important limitation of many SD-OCTA based studies of AMD (70). On the other hand, the study conducted using SS-OCTA by Borrelli et al. demonstrated that the CC FD was greater in intermediate AMD eyes (71). The increase in the CC FD% is thought to be due an increase in the diseased and non-functional choriocapillaris (Figure 1). Lane et al. (72) conducted a comparative study using both SD- and SS-OCTA and demonstrated the superiority of SS technology in quantifying the CC under drusen in early and intermediate AMD (Figure 2). In addition, Zhang et al. reported on a method to compensate for signal attenuation on OCTA using the corresponding strucutural OCT en face slab from the same location (51).
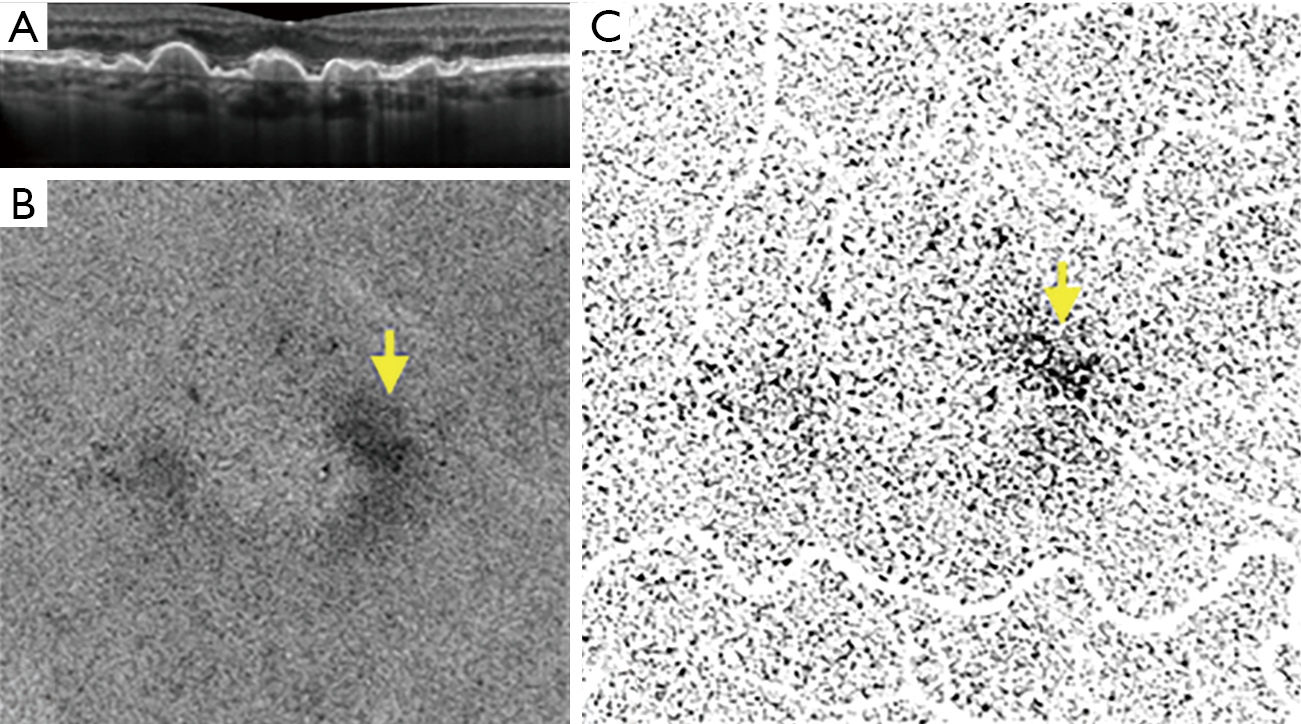
Borrelli et al. (71) also demonstrated that the CC specifically beneath drusen demonstrated an increased CC FD compared to drusen-free region, suggesting a relationship between the status of the choriocapillaris and drusen. In addition, CC FD also appeared impaired in the 150-microns wide region surrounding the drusen, indicating a propensity for capillary segments to be affected in the proximity of an area already functionally impaired (73). More recently, Nassisi et al. investigated choriocapillaris FD in different areas of the macula in eyes with early/intermediate AMD and correlated CC FD% in these regions with the subsequent development or enlargement of drusen (55). New incident drusen or enlargement of existing drusen correlated with those regions where the CC FD appeared more impaired at baseline, suggesting that the location in which drusen develop may not be stochastic, but rather dictated by the status of the underlying CC. Mullins et al. reported that increased sub-RPE deposit density correlates with CC loss and the development of drusen over areas of choroid with ghost vessels (73). In 2019, Braun et al. investigated the correlation between the clinical stage of dry AMD and the global and regional CC perfusion using SS-OCTA (74). The authors found a relationship between the stage of dry AMD and CC perfusion, most prominent in the more peripheral regions of macular 6 mm × 6 mm OCTA scans. We also recently reported a correlation between CC FD% and scotopic microperimetric retinal sensitivity in early and intermediate AMD (75,76). Moult et al. reported an association between drusen-associated GA (DAGA) and CC FD (77). Taken together, these findings appear to highlight the central role of CC in the progression of intermediate dry AMD to nascent GA and successively GA (50,78,79). Our group has compared the CC FD in eyes affected by GA with normal controls and eyes with MNV, finding that CC FD are greater in eyes with GA (49). We speculated that MNV could possibly represent a compensatory response to CC impairment as the “last-attempt” to protect RPE and photoreceptors. In contrast, eyes which progress to GA may represent a more severe stage of the disease with poor and altered compensatory mechanisms (80). One topic of debate with regards to the CC FD in eyes with GA is whether the CC “flow deficits” represent real absence of flow (i.e., loss or closure of the CC), or simply reduced flow below the detection threshold. Several studies have published alterations in the CC FD in eyes with GA in the area surrounding the GA lesion, in which the RPE does not show detectable atrophic changes yet (50) (Figure 3). Nassisi et al. reported an increased CC FD in the zone immediately surrounding the GA lesions, suggesting these areas could be relevant to the progression of GA (50). In a subsequent study, Nassisi et al. investigated the correlation between CC FD within the peri- and para-atrophic region surrounding the GA lesions and the yearly growth rate (yGR) of GA, reporting a statistically significant correlation (78). More recently, Alagorie et al. analyzed the CC FD in eyes with GA in different locations using multiple concentric rings surrounding the GA lesions and correlated the FD% with the yGR of GA (79). Only the CC FD% in surrounding rings within 500 microns of the GA border were correlated with the yGR of the lesion. In contrast, Thulliez et al. found the strongest correlation between choriocapillaris FD and rate of progression when considering the entire scan area outside of the GA lesions, rather than just the zone immediately surrounding the GA (81). Regardless, these studies highlight the important role of the choriocapillaris in the progression of dry AMD.
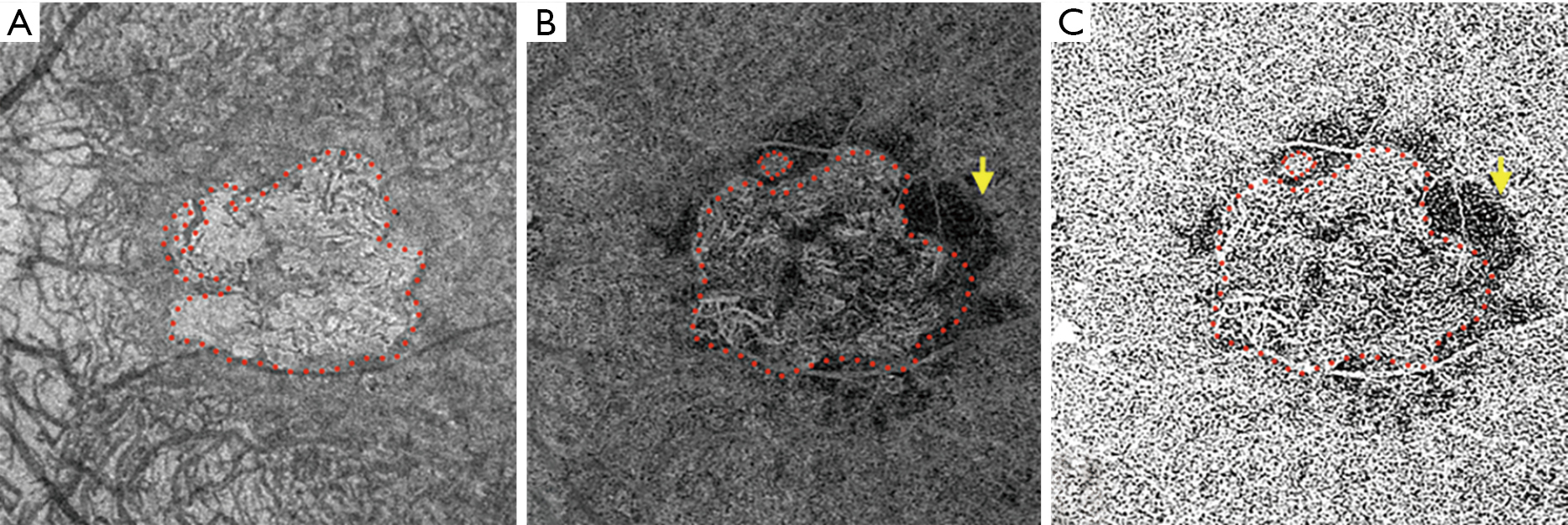
Although OCTA imaging has provided new insights into the CC, there are several limitations that must be considered (82,83). First, the quality of the images obtained by OCTA can be degraded by the presence of artifacts, which can impair the accurate interpretation and quantification of the choriocapillaris (83). In eyes affected by dry AMD, the presence of drusen and Z-axis micro-motion can produce projection artifacts translated into a false positive flow signal (pseudoflow), which could impact quantitative analysis of choriocapillaris (84). OCTA manufacturers have developed various algorithms to reduce or eliminate the projection artifacts, though they remain to be validated against histology in the setting of disease. OCTA instruments are also equipped with proprietary algorithms to compute and measure the Imaging Quality Index (IQI) and the Signal Strength Index (SSI) of the images, but different manufacturers have different acceptable minimum-quality standards, making it difficult to compare between devices. Although, Holmen et al. (85) reported that these indices show poor specificity and high sensitivity, the study was carried out only on eyes with diabetic retinopathy. On the other hand, Al-Sheikh et al. (86) have published on the impact of image quality on OCTA quantitative measurements, and recommended maintaining consistent image quality in order to allow reliable quantitative measurements using OCTA. Uji et al. introduced the concept of “averaging” multiple en face OCTA images to reduce granularity and improve visualization for more reliable quantitative CC measurements (87,88). Another important consideration is that quantitative CC parameters may be affected by the location of the slab segmentation and small differences in the slab selection can alternate the results (62,66,89). Segmentation slabs close to the RPE can be susceptible to segmentation errors, and in fact in a recent study by Byon et al. the most repeatable CC FD% was found with a deeper slab 10-μm thick, located 21 μm below the Bruch membrane (89). It is not clear, however, whether a deeper slab can serve as an adequate surrogate for the CC slab or whether it is better termed an inner choroidal slab. Finally, although methods have been proposed to compensate for signal attenuation due to overlying structures such as drusen, these methods remain to be validated, and could potentially introduce other unintended artifacts. In addition, the selection of the local window radius can significantly impact the CC quantitative results when using local thresholding algorithms such as Phansalkar’s method (66). The selected radius needs to be tuned to the resolution and dimensions of the image rather than a specific number of pixels. Despite these many limitations, OCTA has contributed richly to enhancing our knowledge regarding choriocapillaris alterations in dry AMD.
In summary, OCTA can be used to visualize and quantify CC impairment in dry AMD. The choriocapillaris may play a primary role in the pathogenesis of the non-neovascular AMD and warrants longitudinal prospective studies to further elucidate the pathophysiologic sequence. The CC FD may also provide a useful biomarker to assess the stage or severity of AMD, and to predict its progression. As such, OCTA-derived CC metrics may be useful in future therapeutic clinical trials for AMD.