Abstract: Idiopathic intracranial hypertension (IIH) is a condition in which elevated pressure in the cerebrospinal fluid can lead to optic nerve head (ONH) dysfunction and subsequent visual impairment. Physicians are currently limited in their ability to monitor and manage this condition, as clinical symptoms and exam findings are often delayed in response to changes in intracranial pressure. In order to find other biomarkers of disease, researchers are using imaging modalities such as optical coherence tomography (OCT) to observe microscopic changes in the eye in this condition. OCT can create 2-dimensional and 3-dimensional high definition images of the retina of the ONH and has been used to study various conditions such as glaucoma and multiple sclerosis. Numerous studies have used OCT in IIH as well, and they have shown that certain retinal layers and the ONH change in thickness and shape in both the short and long term with intracranial pressure changes. OCT is a promising modality for clinical and scientific evaluation of IIH as it is a noninvasive and practical tool to obtain in depth images. This review will discuss how OCT can be used to assess a patient with IIH, both before and after treatment, along with its limitations and future applications.
Idiopathic intracranial hypertension (IIH) is a condition in which there is high cerebral spinal fluid (CSF) pressure in the subarachnoid space that surrounds the brain and spinal cord [intracranial pressure (ICP)] due to an unknown cause. It affects 1:100,000 individuals annually with a 20 fold higher incidence in young, obese females (1,2). Symptoms of this condition include headache, pulsatile tinnitus, and vision loss, among others. Visual impairment can manifest as enlarged physiologic blind spots in the visual field due to optic nerve head (ONH) enlargement, peripheral vision loss progressing to central vision loss due to optic nerve dysfunction, double vision due to 6th nerve palsy, and/or transient visual obscurations thought to be due to ONH ischemia (this represents a major morbidity of IIH). Optic nerve dysfunction occurs in association with papilledema, which refers to ONH swelling resulting from exposure of ONH axons and their vascular supply to increased CSF pressure in the optic nerve sheath, which is contiguous with the intracranial subarachnoid space (3,4).
Currently, physicians must rely on detecting downstream outcomes of high ICP, such as ONH appearance or visual impairment, to monitor IIH. The Frisén scale describes stages of ONH swelling on ophthalmoscopy, using blurring of the optic disc margins and vascular obstructions by swollen retinal ganglion cell (RGC) axons to classify papilledema (5). A limitation of this qualitative scale is poor inter-rater reliability (6). In addition, changes in papilledema and changes in visual function can take days to manifest after changes in ICP (7), which introduces delays in assessing therapeutic efficacy with risk of unnecessary escalation of therapy. While intracranial opening pressure can be directly measured with lumbar puncture (LP), the morbidity of this procedure precludes routine use for monitoring IIH after the initial diagnosis has been secured. There is a need for better biomarkers of IIH that are reliable and accurately assess current disease state.
Optical coherence tomography (OCT) is a non-invasive imaging method widely used in ophthalmology to provide high-resolution cross-sectional images of the retina. Two-dimensional OCT images are comprised of a series of linear ‘A’ scans, each based on the backreflected light energy from the device laser by structures with different optical properties, assembled next to each other to create a cross sectional image, or ‘B’ scan. Adjacent B-scans can in turn be used to generate volumetric images. OCT depth resolution ranges from 1 to 15 μm (8), depending on the wavelength and imaging strategy employed. It is used clinically to monitor glaucoma (9) and age related macular degeneration (10) among other conditions. Changes in neurological conditions including multiple sclerosis (11) and Parkinson’s Disease (12) are also well described.
OCT has also shown promise for diagnosis and monitoring of IIH as it captures ONH swelling that characterizes papilledema, retinal nerve fiber layer (RNFL) and retinal pigment epithelium/Bruch’s Membrane (RPE/BM) changes that are associated with acute and chronic changes in ICP (13,14). The objective of this review is to describe ICP related changes visible on OCT’s, how these might be applied to diagnosis and monitoring of IIH and directions for further studies.
The RNFL is comprised of retinal ganglion cell axons extending radially from the optic nerve and is visible on OCT as the superficial retinal layer located just beneath the inner limiting membrane. Peripapillary RNFL is assessed on most commercial OCT devices using the ‘circle scan’ which is a cross-sectional image taken along the circumference of an approximately 3mm diameter centered on the ONH. The average thickness of the top retinal layer in this scan pattern is reported as the average RNFL thickness. In normal anatomy, RNFL thickness varies in the quadrants surrounding the optic nerve, decreasing in height from the inferior, superior, nasal, and temporal quadrants (the ISNT rule) (15). In IIH with papilledema, the RNFL layer has been shown to increase in thickness with papilledema compared to controls (16-18), related to axoplasmic stasis causing thickening of the RNFL layer that contains the axons of the swollen RGCs (Figure 1). This increase is often not symmetric, with increase in thickness favoring superior to inferotemporal sites via nasal zone (17). As with the Frisén scale, temporal RNFL thickening is a finding occurring in association with more severe papilledema. On this basis, it has been proposed that RNFL thickness can be used as an indicator of papilledema in IIH. However, there are limitations. Vardanian Vartin et al. found that there was no difference in RNFL height between controls and patients with mild papilledema found on ophthalmoscopy (18). Other studies have found that RNFL thickness can be difficult to measure in patients with a Frisén grade equal to or above 3 (19,20). A possible reason for this phenomenon is that with increased thickness of the RNFL layer in papilledema, there is increased scatter and absorption compared to reflected light on the OCT, making it more difficult to detect the posterior border of the RNFL (21). In these situations, total retinal thickness, also measured using the peripapillary circle scan can be used to assess papilledema.
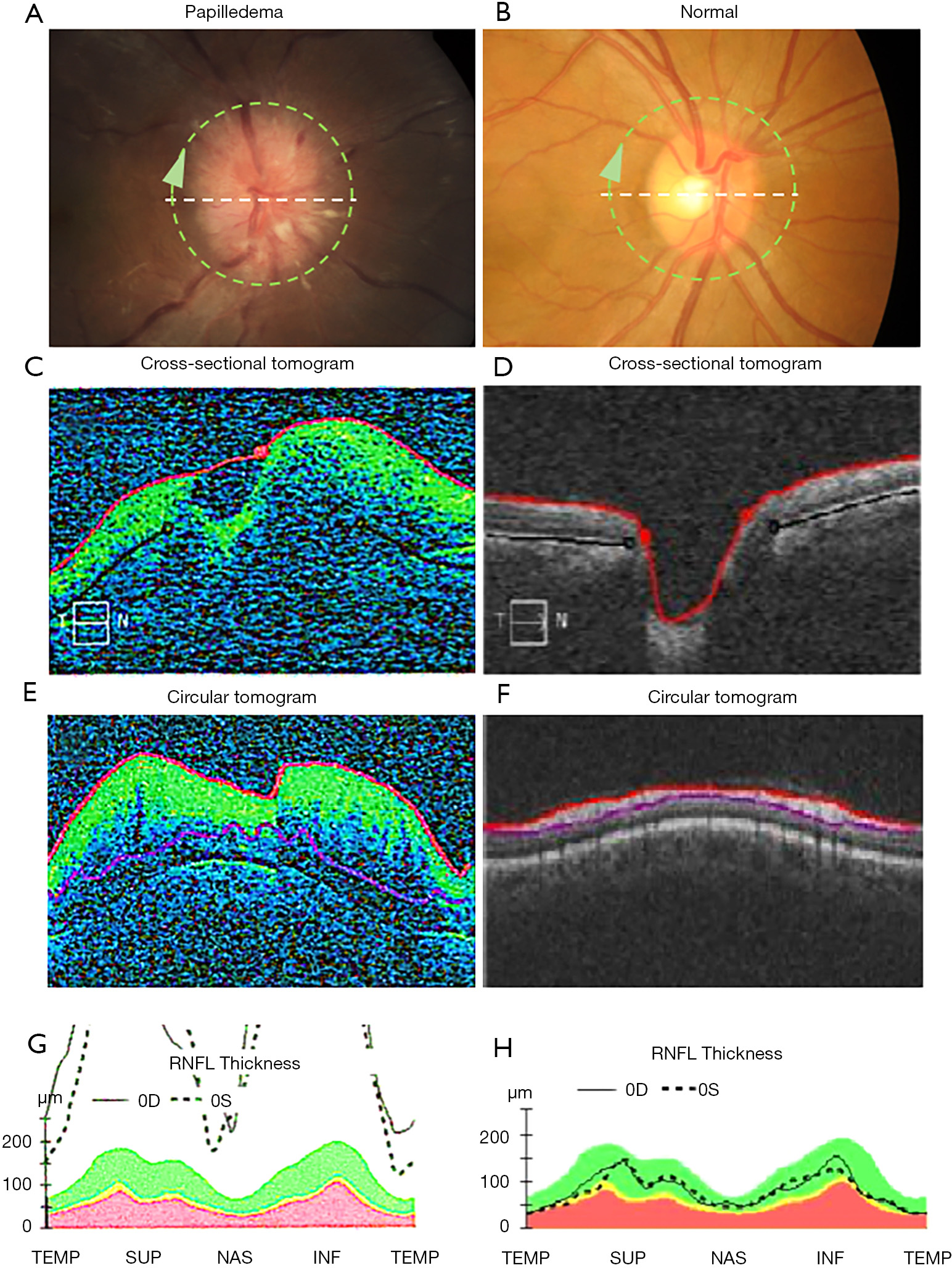
Clinical applications of RNFL thickening associated with papilledema include detection and monitoring of papilledema. RNFL thickness normalizes both in the short and long term with treatment in patients with acute IIH (22). In addition, OCT imaging of the RNFL is correlated with treatment outcomes of IIH. Numerous studies have found that RNFL thickness decreases after treatment to be associated with improvements in visual field mean deviation (21,23). Caution must be taken when attributing RNFL decrease over time to improving papilledema as evolving optic atrophy from RGC injury can cause a similar OCT pattern.
RNFL may be useful for differentiating true ONH edema, including papilledema, from pseudopapilledema due to ONH drusen, as RNFL is thicker in people with papilledema (17,24). Certain regions around the ONH are more effective in differentiating true edema from pseudopapilledema: a few studies found that the nasal sector is most sensitive (24,25), while Carta et al. found that the average and inferior peripapillary quadrants had maximal sensitivity and specificity with a positive predictive value greater than 80% at certain cutoff values (26). Fard et al. found RNFL was thicker in true papilledema vs. pseudopapilledema with 73% sensitivity and specificity. They also compared total retinal volume in concentric rings centered on the optic nerve between the two groups. Their results showed that pseudopapilledema only affects the inner peripapillary ring, while true edema affects the outer ring as well (27).
The ONH is the focus of many studies in IIH, as its appearance and volume are affected by IIH and increased intracranial pressure. The Frisén scale is a qualitative tool used to grade swelling on ONH photographs and exam. While this scale has been shown to have inter-rater reliability to assess papilledema from lack of papilledema, a study by Sinclair et al. demonstrated that it is not as consistent in IIH patients, with only 36% reproducibility amongst raters (6). It is important, then, to develop reliable methods to assess the ONH. Studies support that OCT is a reliable measure to assess the protrusion height and volume of the ONH (28,29). ONH volume, extracted from volumetric OCT scans is correlated with Frisén grade by expert graders. ONH volume measured by OCT has been shown to be increased in patients with IIH and associated with ICP (30), even in those who have a normal RNFL thickness (31). OCT derived measures of ONH volume may be more sensitive to papilledema improvement than the Frisén scale for purposes of monitoring treatment of IIH. Some studies have found that while ONH volume and Frisén scale grading correlated at baseline in patients with IIH, they were not consistent after 6 months of treatment with acetazolamide and weight loss (32,33). Auinger et al. suggested that the lack of correlation with treatment may be due to a floor effect and ordinal scale of Frisén grading compared to the continuous nature of ONH volume measures.
A limitation in utilizing ONH volume in routine clinical practice is that most commercial devices do not automatically provide this measure. One technique to overcome this is to center the macula scan protocol on the ONH in order to leverage the automatic volume calculations associated with these protocols. However, difficulties in segmenting the outer retinal boundary can lead to errors. As with RNFL thickening, caution must be taken when attributing ONH decrease over time to improving papilledema as evolving optic atrophy from RGC injury is independently associated with ONH volume (30).
The retinal pigment epithelium/Bruch’s Membrane (RPE/BM) layer is the most clearly defined outer retinal layer on OCT, located above the choroid. In control patients, the RPE/BM around the optic nerve is V-shaped and angled away from the vitreous as it approaches the neural canal opening, but in patients with papilledema due to IIH, it has been shown on OCT to have an inverted U shape toward the vitreous (34,35) (Figure 2). While inward RPE/BM angulation with elevated intracranial pressure is evident on cross sectional ONH images, its shape is not uniform across radial scan angles, suggesting asymmetric changes in RPE/BM shape with IIH (36). A variety of strategies have been used to assess this shape including measures of angle (35), volume (30), and geometric morphometrics. This latter technique applied principal component analysis to compare shape, which is a geometric feature independent from scale, position, and rotation (34). RPE/BM shape has also been shown to correlate with quantitative ICP (37) and to differentiate papilledema from high ICP (e.g., IIH) from optic disk edema due to other optic nerve injury (e.g., non-arteritic anterior ischemic optic neuropathy) which have RPE/BM angulation away from the vitreous similar to people without ONH edema (34,35,38). Sibony et al. hypothesized that RPE/BM angulation in IIH is due to the translaminar pressure gradient between cerebral spinal fluid (CSF) and intraocular pressure combined with properties of the peripapillary sclera similar to globe flattening (38).
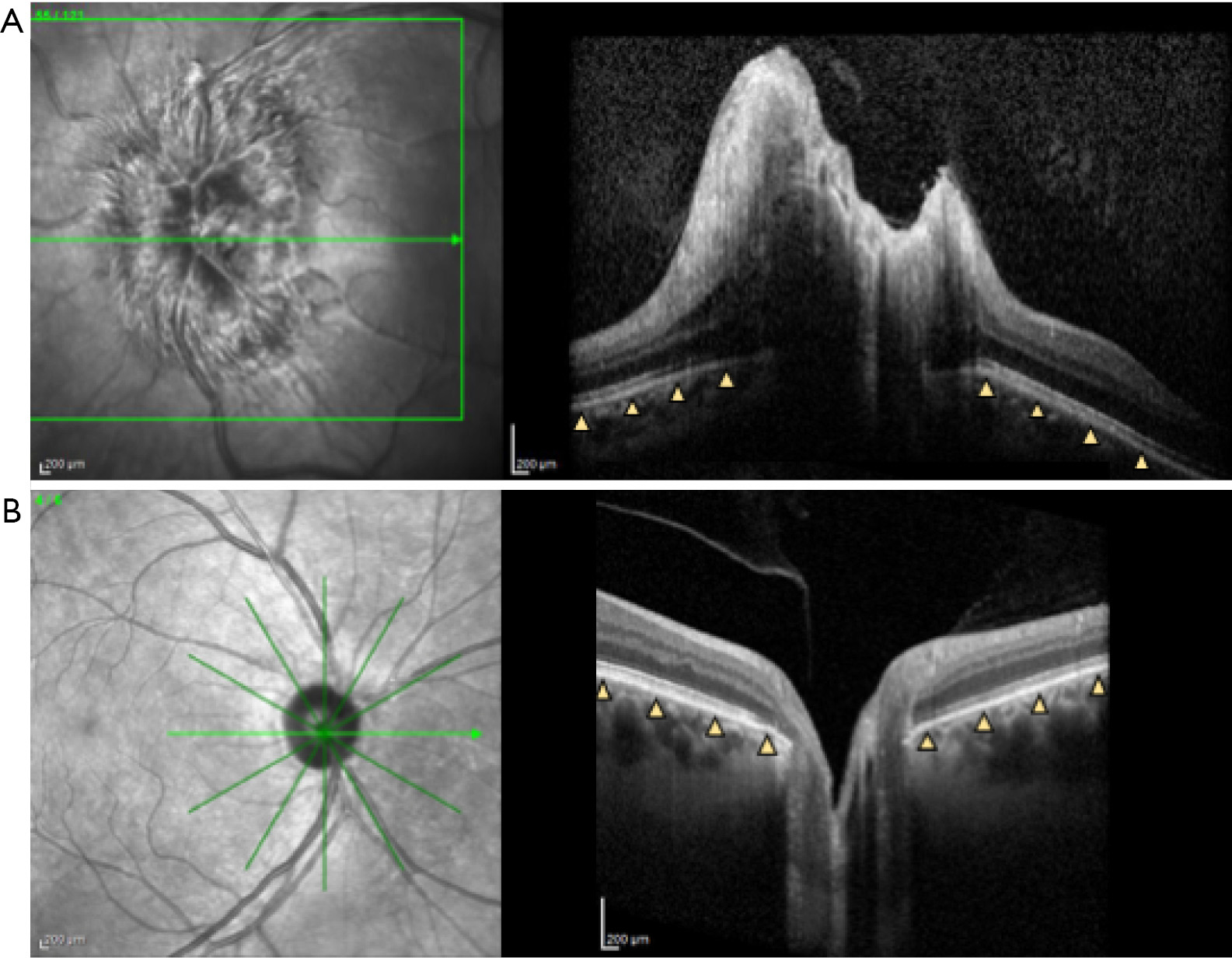
Peripapillary RPE/BM shape changes can be detected following both acute and chronic IIH treatment, as quickly as 1 hour after LP (37,38), and are correlated with decreased RNFL thickness after CSF shunt and medical treatment of IIH (39). However, in patients with prolonged intracranial hypertension and subsequent optic nerve atrophy, improvements in RPE/BM shape were not associated with normalized changes in RNFL. In such cases RPE/BM shape may better serve as a biomarker of increased intracranial pressure as it is independent of optic disc edema (39). In the OCT sub-study of the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT), RPE/BM shape normalized in patients randomized to acetazolamide plus weight management, but did not change in patients randomized to placebo plus weight management alone despite improvements in papilledema in this group. This provides further evidence that RPE/BM shape and ONH edema are independent of one another (13).
A limitation of utilizing peripapillary BM/RPE changes to guide clinical care is the lack of a commercially available algorithm. Research study of these changes has required intensive image processing often with manual components due to difficulty in identify the BM/RPE boundary beneath the swollen ONH (40).
Patton’s (retinal) and choroidal folds can be appreciated on ophthalmoscopic examination of papilledema as concentric curved lines adjacent to the swollen ONH and radial lines extending from the ONH. OCT demonstrates the cross-sectional structure of these folds and is more sensitive to their detection than fundus photography. In the IIHTT, nearly half of subjects in the OCT sub-study had peripapillary superficial retinal wrinkles and/or inner retinal folds (Figure 3), while 10% had choroidal folds (41). The first two were associated with papilledema severity while choroidal folds were related to BM/RPE shape change. A subsequent analysis also characterized outer retinal folds/creases thought to be distinct biomechanical responses to ICP and its effect on the optic nerve (42). OCT can detect peripapillary wrinkles in adduction in people with papilledema who lack them in primary gaze (43). These observations raise the possibility of a diagnostic role of these signs for differentiating papilledema from pseudopapilledema. In the IIHTT, some kinds of folds resolved following treatment, more so in acetazolamide treated subjects, but some persisted (44). These observations advance insights into the effects of ICP and ONH swelling on the eye, but their role in clinical management remains uncertain.
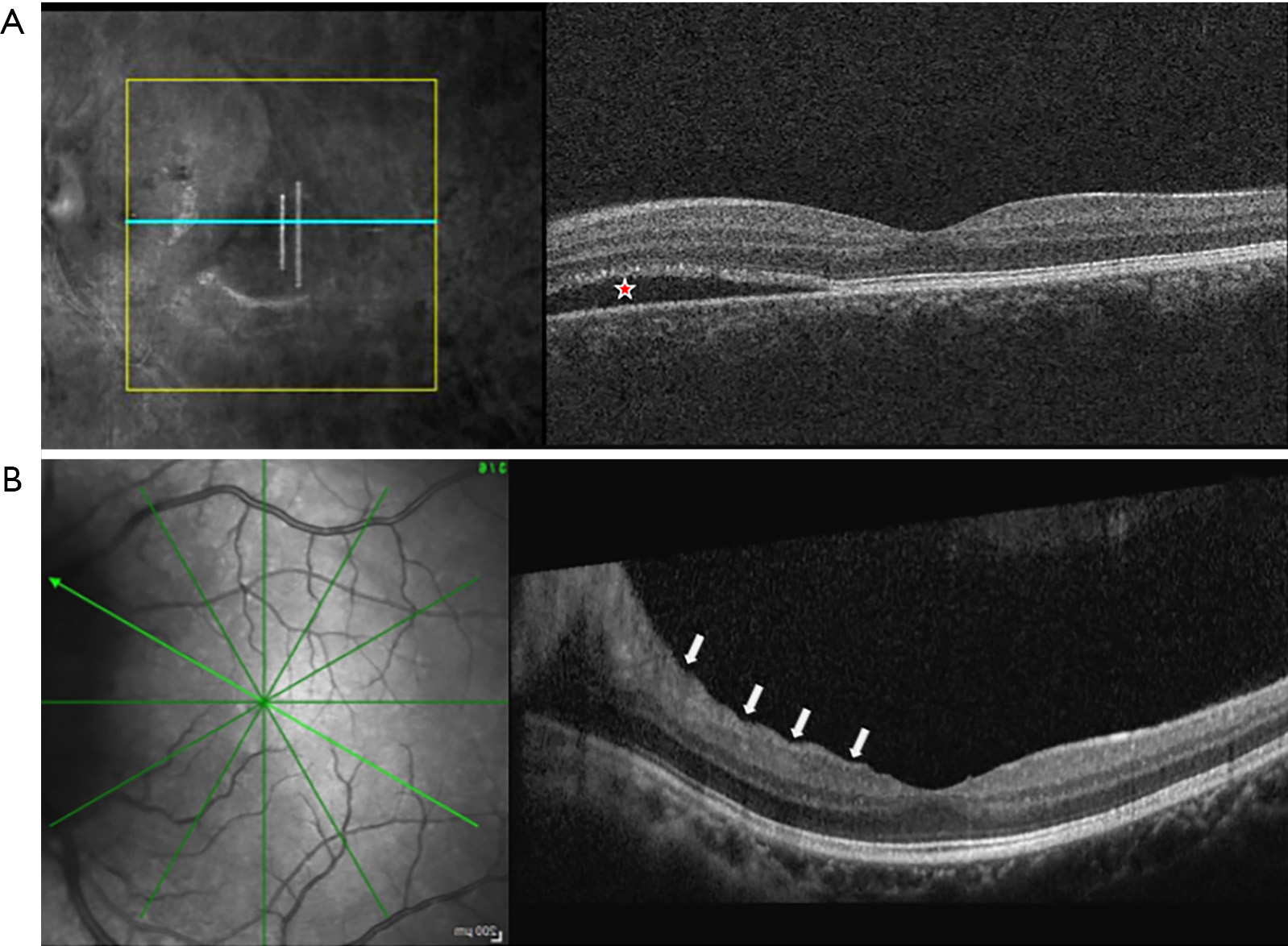
Because RNFL is thickened in association with papilledema it is often not useful to detect retinal ganglion cell atrophy. However, decreased macular ganglion cell complex average thickness or volume, measures of the superficial retinal layers containing the retinal ganglion cell bodies and other structures from OCT scans centered on the macula, are an indicator of early optic nerve atrophy in IIH (45). Atrophy detection can be reduced in severe papilledema when ganglion cell axon thickening extends into nasal portions of the macula scan. Macula ganglion cell complex measures correlate with future visual field measures (46), suggesting that they may play a role in predicting poor visual outcomes in IIH, and identifying patients at need for more aggressive therapy (47).
Cross sectional OCT scans centered on the macula are also useful for identification of non-retinal ganglion cell contributions to central vision loss such as subretinal fluid, often extending from the optic nerve (Figure 3), which can cause outer retinal injury, chorioretinal folds and choroidal neovascularization (47).
Algorithms that detect movement during OCT detection have been optimized to generate maps of blood flow on OCT images, known as OCT angiography (OCTA), which is now offered on multiple commercial OCT devices. In addition to qualitative patterns of perfused blood vessels, these images allow calculation of vascular density in different retinal layers. Limitations include lack of differentiation between venous and arterial vessels as well as shadowing of deeper vessels by superior vessels, leading to incomplete imaging of deeper vessels.
OCTA has been applied in cross-sectional studies of papilledema. Qualitative features detected using OCTA of the ONH include dilation and tortuosity of large vessels and tangling/curling of capillaries, as opposed to capillary drop out seen in anterior ischemic optic neuropathy (48). Quantitative analysis of vessel density around the optic nerve, including all vessels, found increases in papilledema compared with ischemic and inflammatory causes of pathologic ONH edema (49), but decreases compared to controls (50,51). When large vessels were excluded from analysis peripapillary capillary density was similar to controls, suggesting that papilledema has differential effects on different levels of the vasculature. Macular OCTA densities were similar in papilledema eyes without GCC thinning compared to control eyes, which distinguished them from NAION eyes without GCC thinning in which OCTA densities were reduced (52). At this time the role for OCTA in diagnosing and monitoring IIH is unclear, though it shows promise in providing insight into the pathophysiology of diseases impacting the ONH.
While OCT metrics show promise as quantitative markers of optic nerve and other retinal changes in IIH, there are still challenges that must be overcome to optimize use of this imaging modality to diagnose and monitor patients in clinical settings. Image quality and segmentation is a significant challenge as ONH edema can lead to reduced distinction between peripapillary retinal layers necessary to analyze the RNFL, shadowing of RPE/BM layers, identification of which is necessary to analyze shape and shadowing of deeper retinal capillaries by superficial vessels and swollen tissue. Iverson et al. suggested that peripapillary Bruch’s mebrane (pBM) automatic segmentation programs may inaccurately identify the pBM end point when it is close to the cup border, when the border tissue of Bruch’s membrane extends past the RPE or when the signal intensity is diminished from shadows generated by overlying vasculature in non-swollen optic nerves (53). Although this can present limitations to using OCT in a clinical setting, there are promising studies that show that consensus review and creating a set of guidelines for segmentation improves agreement between raters (36,54). There are numerous commercial and research segmentation programs with varying levels of accuracy. One strategy is to exclude areas of uncertainty from image analysis (30). Strategies to improve image quality involve scan protocol (e.g., averaging, focus), device design and image processing, for example eliminating blood vessel shadows and increasing contrast between layers to make automatic segmentation programs more accurate (55).
OCT is a relatively new clinical imaging technique, with the first commercial clinical instrument becoming available in 1996 with widespread clinical ophthalmic use by 2005. OCTA was not available commercially until 2014, with quantitative analysis lagging behind (56). Studies to date have illustrated many potential OCT derived metrics that have potential application to diagnosing and managing IIH. In the author’s experience it is widely used to compare optic nerve appearance using ONH scans and to detect ganglion cell injury using macular scans. Additional larger studies are needed to establish the role for imaging of folds and vasculature. Commercially available scan protocols and analysis algorithms are needed to facilitate broad adoption.
OCT shows promise as an important imaging technique to supplement other clinical data in the diagnosis and monitoring of IIH. It is currently in use in conjunction with other imaging modalities and clinical features to identify early changes in the ONH and retina both before and after treatment of intracranial hypertension. It is also an important research tool with application to understanding the pathophysiology of papilledema. As more research is done to improve image quality and segmentation, and large human studies are completed, the role of OCT in the management of IIH will grow.