Abstract: Focal intraretinal alterations have been studied to advance our understanding of the pathology of neurodegenerative diseases. The current literature involving focal alterations in the intraretinal layers was reviewed through PubMed using the search terms “focal alteration”, “region of interest”, “optical coherence tomography”, “glaucoma”, “multiple sclerosis”, “Alzheimer’s disease”, “Parkinson disease”, “neurodegenerative diseases” and other related items. It was found that focal alterations of intraretinal layers were different in various neurodegenerative diseases. The typical focal thinning might help differentiate various ocular and cerebral diseases, track disease progression, and evaluate the outcome of clinical trials. Advanced exploration of focal intraretinal alterations will help to further validate their clinical and research utility.
With the advent of optical coherence tomography (OCT) in the 1990s (1,2), the evolved image resolution and robust segmentation of the intraretinal layers in volumetric data allowed for detailed analysis of retinal structure (3,4). The most widely used segmentation involves the following layers: retinal nerve fiber layer (RNFL) (5,6), which contains unmyelinated axons, ganglion cell-inner plexiform layer (GCIPL), which contains retinal ganglion cell (RGC) bodies (7,8), and the ganglion cell complex (GCC), which includes RNFL and GCIPL (9). Though definitions vary in different OCT devices, these retinal layers have been suggested to be structural image biomarkers, which have been used in monitoring disease progression and aiding in diagnostics of neurodegenerative diseases (5,10,11). The majority of these published studies used the average thickness of certain areas such as the macula. However, the distribution of the neural fiber and ganglion cells in the retina is not even (12-16). For example, using Zeiss OCT, nasal and superior quadrants of GCIPL are thicker compared to the temporal and inferior quadrants in normal healthy subjects (12). Furthermore, the uneven distribution of the intraretinal layers has been demonstrated in normal healthy subjects using ultra-high-resolution OCT (UHR-OCT) and commercial segmentation software (Orion software) (13,14,16). Moreover, based on previous histological studies, there is a selective vulnerability of RGCs to various neurodegenerative disorders (17). Parasol RGCs and related magnocellular pathways are more involved in the pathogenesis of Alzheimer’s disease (AD) (18). The parasol RGCs are usually located in the peripheral retina. More interestingly, the prominent Aβ deposition has been demonstrated in the superior-temporal quadrant in flat-mounted retinas (18). Patients with AD had inferior visual field loss (17,18). The midget RGCs and the parvocellular pathway, located in the papillomacular bundle (center of the macula), are more involved in mitochondrial optic neuropathy (19,20). Hence, the commonly used average thickness of these intraretinal layers may not be sensitive enough to reveal the focal thickness alterations (21-24), which limits further improvement of these neural biomarkers in diagnostic powers.
Because of the limitations of the thickness parameters averaged from the area, further analysis of sectional areas has been applied (25-27). Sectional thickness analysis using arbitrary partitions, such as the Early Treatment Diabetic Retinopathy Study (ETDRS) partition, hemisphere partition (16) and Zeiss elliptical partition (21), appears to improve the refinement of thickness alterations and provides a better understanding of the pathologic locations. The improved analysis further strengthens the establishment of correlations between neurodegeneration and clinical manifestations, which provides better biomarkers for monitoring treatment efficacy in clinical trials (25-27). However, some studies have pointed out that these arbitrary partition methods often define the sections of RNFL and GCIPL, which may not be sufficient to observe early change, especially focal thickness reduction, which may not fall into any predefined partition (13,14,16,28). Hence, beyond the traditional partition method, some studies used the freedom of self-defined regions of interest (ROI) to describe the focal thickness alteration change in neurodegenerative diseases in an attempt to find important associations with clinical manifestations such as visual acuity, visual field, contrast sensitivity and disability (14,25,29,30). However, without direct visualization of the thickness map alteration, exploration of the most profound alteration may miss the target, although the relations between the thickness of the ROI and clinical manifestations may provide some critical information.
In this review, we summarize focal thickness alterations in different regions of the intraretinal layers in normal aging and several central nervous system neurodegenerative diseases, which involve slow progressive loss of neurons in the central nervous system, such as AD and Parkinson’s disease (PD). Current literature involving focal alterations in the intraretinal layers were reviewed through PubMed using the search terms “focal alteration”, “region of interest”, “optical coherence tomography” with “glaucoma”, “multiple sclerosis”, “Alzheimer’s disease”, “Parkinson disease”, “neurodegenerative diseases” and other related items. The clinical implications of these focal thickness alterations and future research directions were discussed.
The most commonly used OCT devices in the clinic provide thickness maps of the GCIPL, GCC and peripapillary RNFL (pRNFL) (5,31,32). The benefits of these approaches are their availability and ease of assessment, which do not require further processing of the OCT scans for further interpretation. The drawbacks are as follows: First, clinical reports of OCT scans do not provide thickness information on other intraretinal layers. Second, the visualization is often limited to each scan and cannot be averaged for further visualization of a group of eyes. Third, the preset partitions, such as the Zeiss elliptical partition and ETDRS partition, cannot be modified, which limits redefining the ROI for detecting focal thickness alterations.
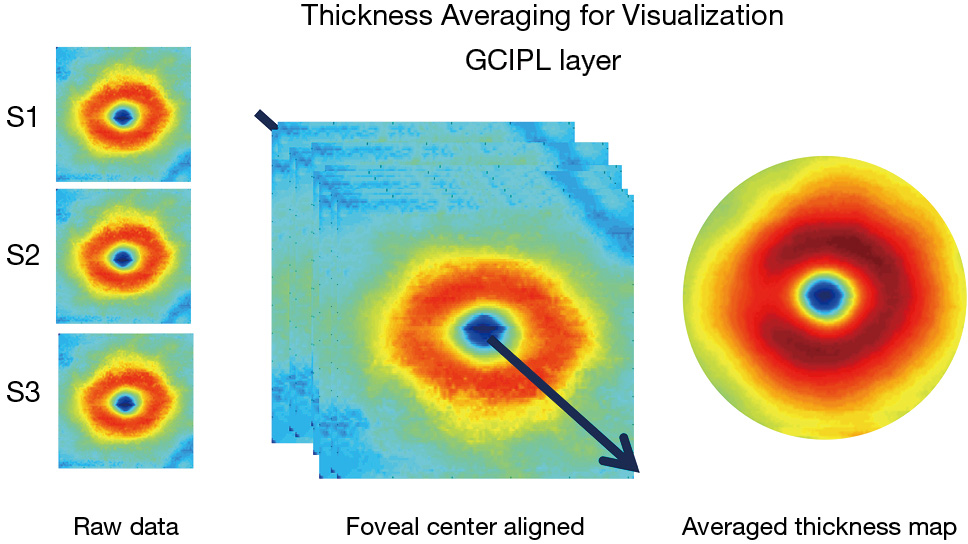
The advancement of retinal segmentation provides a solution to the automated segmentation of the intraretinal layers, which opens the door for visualization of the thickness maps. Chen et al. reported three-dimensional segmentation, which can segment the retina into 11 intraretinal layers and creates a visualization of these segmented layers (3,33). Furthermore, using commercially available segmentation software (Orion, Voxeleron LLC, Pleasanton, CA, USA), up to seven layers can be segmented in the three-dimensional dataset (13-15,34). To visualize the thickness maps of the intraretinal layers for a special group, averaging of each thickness map is required with access to the raw segmented thickness data. Mwanza et al. reported a GCIPL thickness map averaged from 47 normal eyes to demonstrate the thickness contour about the fovea (12). Generating the average thickness requires alignment of the foveal center as an alignment landmark (Figure 1). Of note, the study by Mwanza et al. only segmented the GCIPL and demonstrated the uneven distribution of GCIPL in normal eyes (12). Similar approaches were conducted by our group using UHR-OCT and Orion software (13-16) (Figure 2). These studies segmented six intraretinal layers and visualized focal thickness alterations in patients with AD (15) and multiple sclerosis (MS) (13,14) by calculating the difference of the average thickness maps between the diseased eyes and normal controls (Figure 3). This approach provides a practical method of searching for more sensitive structural biomarkers in neurodegenerative disorders (13-15). The Orion software has been adapted in commercial OCT devices such as Zeiss Cirrus HD-OCT (with special data exportation) (35,36), Topcon 3D-1000 (Topcon Medical Systems, Inc., Oakland, NJ, USA) (34) and Spectralis SD-OCT (Heidelberg Engineering, Heidelberg, Germany) (37). It could be speculated that the visualization of focal thickness alterations can be performed using these commercial OCT devices and the Orion software (34-37).
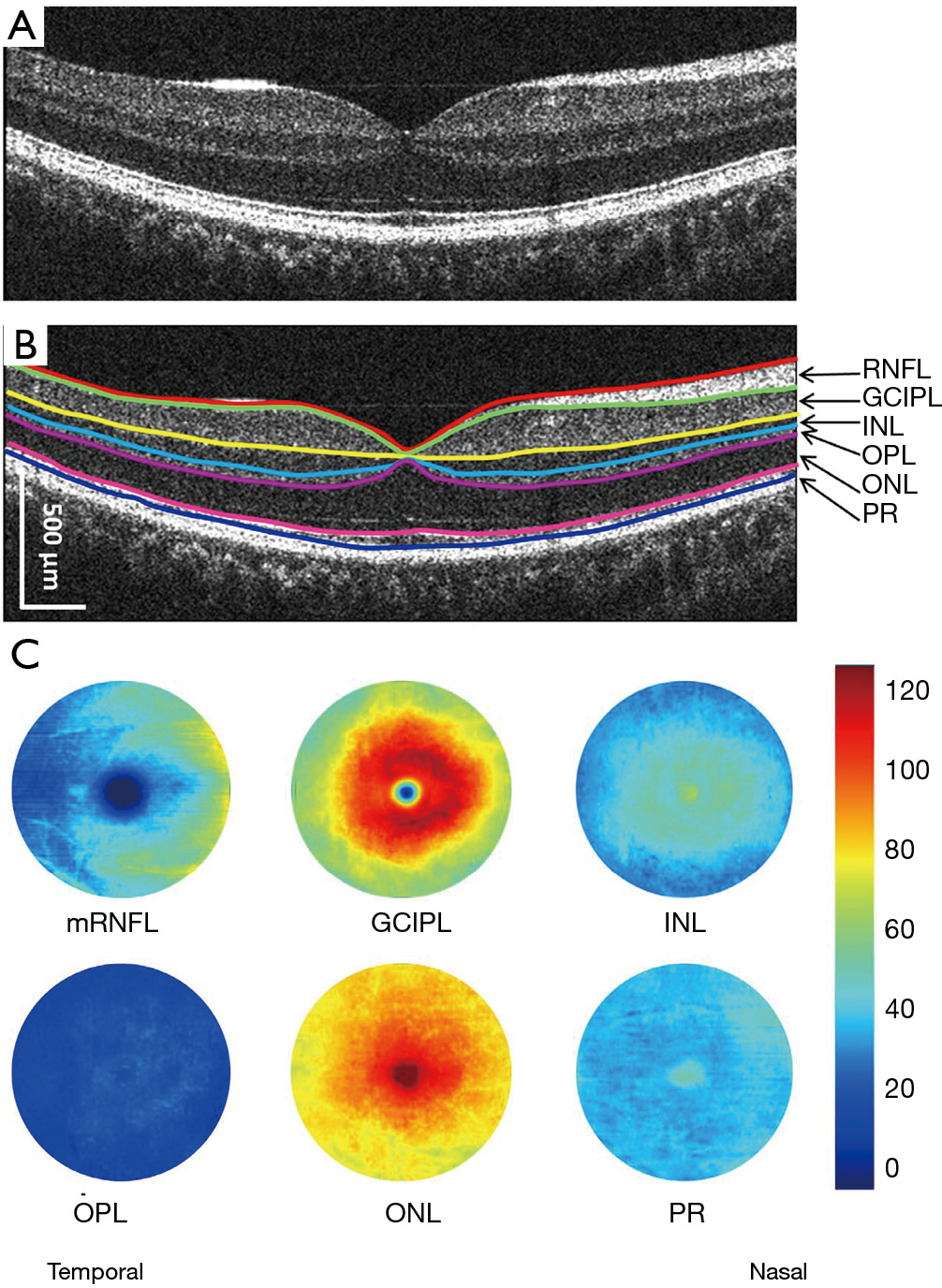
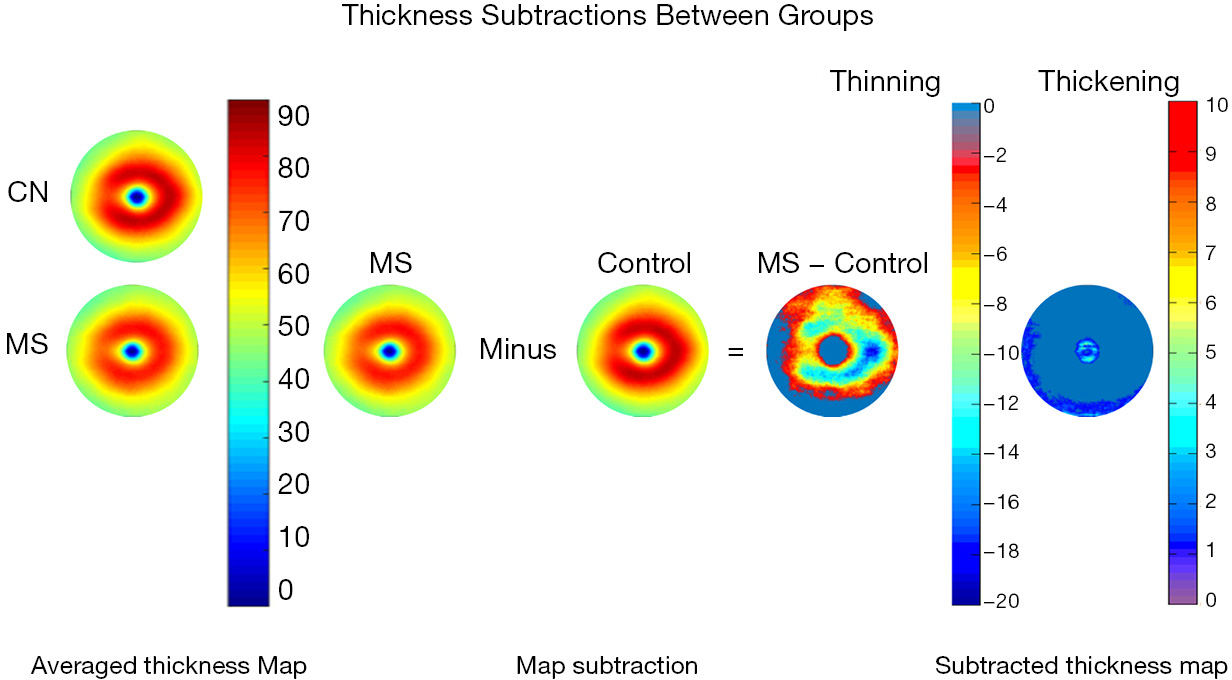
With these recent developments in OCT and segmentation, focal thickness alterations in the intraretinal layers may help determine the most vulnerable region during aging and in certain neurodegenerative diseases, including glaucoma, Leber’s hereditary optic neuropathy (LHON), MS and its related optic neuritis (ON), mild cognitive impairment (MCI), AD and PD (Table 1). The focal thickness reduction zone in the intraretinal layers generally may represent the area that is most seriously affected by certain diseases. These characteristic patterns may also provide disease-specific information and better discrimination powers (38,41,42).
| Authors | Disease | No. of eyes | OCT device | Analysis tool | Thinning pattern | AUC | Map |
|---|---|---|---|---|---|---|---|
| Hu |
Multiple sclerosis with optic neuritis |
30 |
Custom-built UHR-OCT | Orion software | U zone | 0.97 | |
| Shi |
Multiple sclerosis without optic neuritis |
47 patients |
Custom-built UHR-OCT | Orion software | M zone | 0.77 | |
| Lee |
Glaucoma |
67 |
Cirrus HD-OCT | Built-in software | Temporal raphe sign | 0.811 | |
| Chen |
Preperimetric glaucoma |
67 |
SD-OCT | Built-in software | Inferotemporal | 0.784 | |
| Lee |
Ethambutol-induced optic neuropathy |
28 |
Cirrus SD-OCT | Built-in software | Inferonasal | 0.833 | |
| Sharifipour |
Glaucoma |
101 |
Cirrus HD- OCT | Built-in software | Inferotemporal | 0.944 | |
| Yum |
Optic chiasmal compression by a pituitary adenoma |
46 |
Cirrus HD-OCT | Built-in software | Inferonasal, superonasal | 0.965, 0.958 | |
| Shao |
Alzheimer’s disease |
25 |
Custom-built UHR-OCT | Orion software | Inner superior | – | |
| Mild cognitive impairment |
24 |
Custom-built UHR-OCT | Orion software | Outer superior | – | ||
| Shin |
Primary open-angle glaucoma | 292 | Cirrus HD-OCT | MATLAB software | Inferotemporal region (250°–339°) at 2.08 mm from the fovea | – | |
| Balducci |
Acute Leber’s hereditary optic neuropathy |
6 |
Cirrus HD-OCT | Built-in software | Superonasal | 0.9545 | |
| Acute Leber’s hereditary optic neuropathy |
6 |
Cirrus HD-OCT | Built-in software | Inferonasal, superonasal | 1.0, 1.0 | ||
| Cheung |
Alzheimer’s disease |
100 |
Cirrus HD-OCT | Built-in software | Inferior | 0.722 | |
| Mild cognitive impairment |
41 |
Cirrus HD-OCT | Built-in software | Inferotemporal | 0.688 |
GCIPL, ganglion cell-inner plexiform layer; OCT, optical coherence tomography; AUC, area under the curve; UHR-OCT, ultra-high-resolution OCT; S, superior; I, inferior; ST, superotemporal; SN, superonasal; IT, inferotemporal; IN, inferonasal; II, inner inferior; IS, inner superior; OS, outer superior; OT, outer temporal; ON, outer nasal; OI, outer inferior.
Aging increases the likelihood of neurodegeneration in the brain and retina. Age plays a role in the onset of AD, PD, and other neurodegenerative diseases (45). Studying the effect of age on the intraretinal layers improves our understanding of neurodegeneration during normal aging and disease-related retinal morphology changes. The alteration of the intraretinal layers during aging has been well-studied, and annual decline rates provide a reference for disease conditions (46-51). Many OCT studies have reported age-related thickness alteration of the retinal neural layers (46-51). In a study with 121 subjects, the pRNFL was found to decrease by 0.37 μm/year with the greatest decrease in the lower quadrant (0.58 μm) (46). Hammel et al. found that the mean rate of average GCIPL thickness reduction was 0.57 μm/year (47), whereas the average decline rate of the macular GCIPL thickness was reported as 0.12 μm/year in another study (48). In previous studies, Harwerth et al. suggested that 15% of RNFL thickness loss, 50% loss of axons and 40% reduction of axon density in RFNL can occur in a 70-year lifespan (52). Wei et al. reported that, during a period of six decades, the RNFL was 0.35% (0.13 μm) thinning per year and 0.21% (0.14 μm) per year in the GCIPL layer (53). A longitudinal study showed that, over 40 years, the ganglion cells defined as GCC thickness decreased 0.25 μm per year and RNFL decreased 0.21 μm per year (54). Previous studies showed that the inner annulus (IA) part of macular GCIPL was most correlated with age (49-51) (Wang JH, et al., IOVS 201;59: ARVO, E-Abstract 1102). Because the Zeiss elliptical partition mainly covers the IA of the retina, all sectors showed reduced thickness with age (12,48). The variation of the annual thinning rates is mainly due to the differences of the measurement areas of the unevenly distributed intraretinal layers. Other factors to explain the variations include different study cohorts and different OCT devices and segmentation methods.
In contrast to the thinning of the inner retinal layers, age-related thickening was found in some sublayers of the outer retina. Several studies found the thicknesses of the outer plexiform layer (OPL), outer photoreceptor segment (OS), and photoreceptor complex (PR) were positively correlated with age (51,53). This finding can be illustrated by histopathological studies because the density of cone and RPE cells showed no significant loss in the fovea of the eye. Furthermore, the accumulation of residual bodies and loss of melanin granules were found in the retinal pigment epithelium-Bruch’s membrane (RPE-BM) (55,56). These age-related intraretinal layer thickness changes may be mainly due to selective loss of neurons and axons with aging, as found in histology studies (57,58).
Glaucoma, a leading cause of visual impairment, is a group of diseases with multifactorial etiology defined by atypical optic neuropathy accompanying structural damage of RGCs and their axons, resulting in visual field damage and blindness (59,60). Accurate diagnosis is crucial for vision preservation (60). However, it is difficult to differentiate between glaucomatous optic neuropathy (GON) and nonglaucomatous optic neuropathy (NGON) even for a well-trained ophthalmologist (61). Thus, alterations in focal intraretinal layer thickness are useful in diagnosis, and clinical management.
The evolution of OCT with better resolution, faster scanning speed, and advanced imaging patterns improves the reliability of OCT measurements (62). However, in advanced stages of glaucoma, RNFL thinning has a “floor effect”, which may not be sensitive enough to monitor the disease progression (62). Meanwhile, current OCT structural parameters cannot provide differential structural measurements between normal and early glaucomatous eyes (62). OCT-measured pRNFL thickness has been found to have the ability to differentiate between normal and glaucomatous eyes (62,63).
In addition to the thickness measurement of the RNFL, the macular GCIPL layer is another way to monitor glaucomatous progression and detect early glaucoma (38,64). Early involvement and progressive damage of RGCs occurs in both GON and NGON (38,64). Chen et al. found that thickness reduction in the inferior temporal (IT) sector in the Zeiss elliptical partition had the largest discrimination power [area under the receiver operator curve (AUROC) =0.794] to differentiate preperimetric glaucoma eyes from normal eyes (39). Sharifipour et al. reported that the same IT thinning pattern in the Zeiss elliptical partition had an AUROC of 0.944 to detect eyes with early glaucoma (41). These measurements mentioned above were based on commercial OCT with built-in thickness analysis.
By using custom-built retinal thickness measurement software and an improved analysis algorithm, some typical OCT image biomarkers were found (29,43). By subtracting the average RNFL and GCIPL thicknesses of normal eyes from glaucoma eyes, Hood et al. found a pattern located at the inferior area associated with greater visual field loss, which may represent greater vulnerability of the inferior retinal region (29). Using OCT Guided Progression Analysis (GPA) in GCIPL analysis, Shin et al. found a similar area located in the IT region (250°–339°) at 2.08 mm from the fovea, which had the most frequent GCIPL thinning (43). Lee et al. found a temporal raphe sign of GCIPL that can be used to differentiate glaucomatous from nonglaucomatous GCIPL thinning (38).
A specific pattern of retinal thickness layer reduction was also found in LHON, a subacute neurodegenerative blinding disorder characterized by selective loss of RGCs (19). Balducci et al. found that the natural progression of LHON followed a specific pattern of reduction in macular GCIPL located in the inferior nasal pattern (44). The specific pattern changes in macular GCIPL in LHON may indicate that the papillomacular bundle is primarily involved in LHON. The thinning pattern of GCIPL thickness of LHON follows a centrifugal and spiral pattern in line with the anatomic distribution of the papillomacular bundle fibers (44,65). Specifically, nasal focal thinning of GCIPL at the presymptomatic stage before the diffuse thinning of GCIPL was observed in LHON (44).
MS is an autoimmune neurodegenerative disorder affecting the central nervous system (66). Eighty percent of patients with longstanding MS also experience deficits in visual function (67). ON is an inflammatory optic neuropathy that affects many patients with MS at some point during their disease progression (68). OCT is regarded as an alternative to magnetic resonance imaging (MRI) for monitoring MS (69). Thinning of the pRNFL and GCIPL measured by OCT is considered as an ocular biomarker of the central nervous system in MS (70,71). The thickness reductions of pRNFL and GCIPL have been used in MS clinical trials (5,72). However, although the thickness reductions of pRNFL and GCIPL were significant among groups of patients with MS without a history of optic neuritis (MSNON), MS with a history of ON (MSON) and matched normal controls had longitudinal changes of these neuronal layers of ~1 μm per year. This poses difficulty in following up on disease severity and therapeutic efficacy (5,23), which inspired the exploration of more sensitive retinal structural markers for monitoring neurodegeneration compared to the average thickness reduction of pRNFL and GCIPL. Detailed analysis of focal thickness alteration may meet the challenge.
Shi et al. used UHR-OCT and automatic segmentation software to visualize topographic thickness alterations of intraretinal thickness in patients with relapsing-remitting MS without a history of ON (14). The visualized thickness map of GCIPL showed a profound thickness reduction zone, named “M Zone”, short of the “MS thinning Zone of the GCIPL” (Figure 4). It was a circling zone located at nasal ~2 mm and inferior 0.42 mm from the fovea. The “M zone” was found to be the most profound alteration compared to any sectors partitioned using the ETDRS partition. The discrimination power (AUROC =0.75) was second to pRNFL (AUROC =0.79) in differentiating eyes with MS and no ON. Furthermore, the focal thickness alteration in the M Zone had good correlation with MS-related disability and visual dysfunctions. The authors suggested that the focal thinning zone (i.e., the M Zone) can be an imaging biomarker to monitor neurodegeneration in patients with MS. The finding also echoes the thinning of temporal pRNFL compared to other zones in MS patients without ON (73). It is also consistent with previous histological studies reporting midget RGCs and the parvocellular pathway located in the papillomacular bundle, which are more selectively damaged (18,74,75).
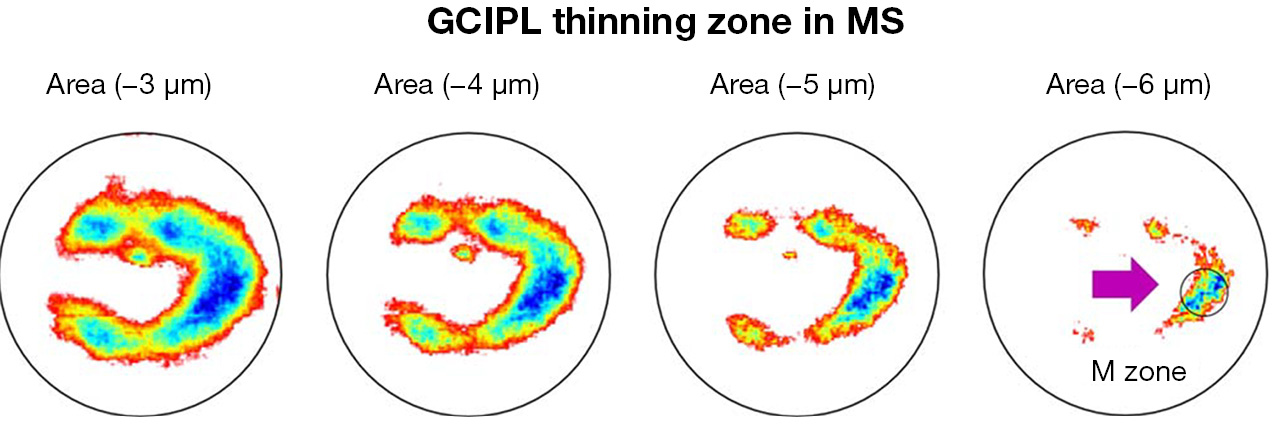
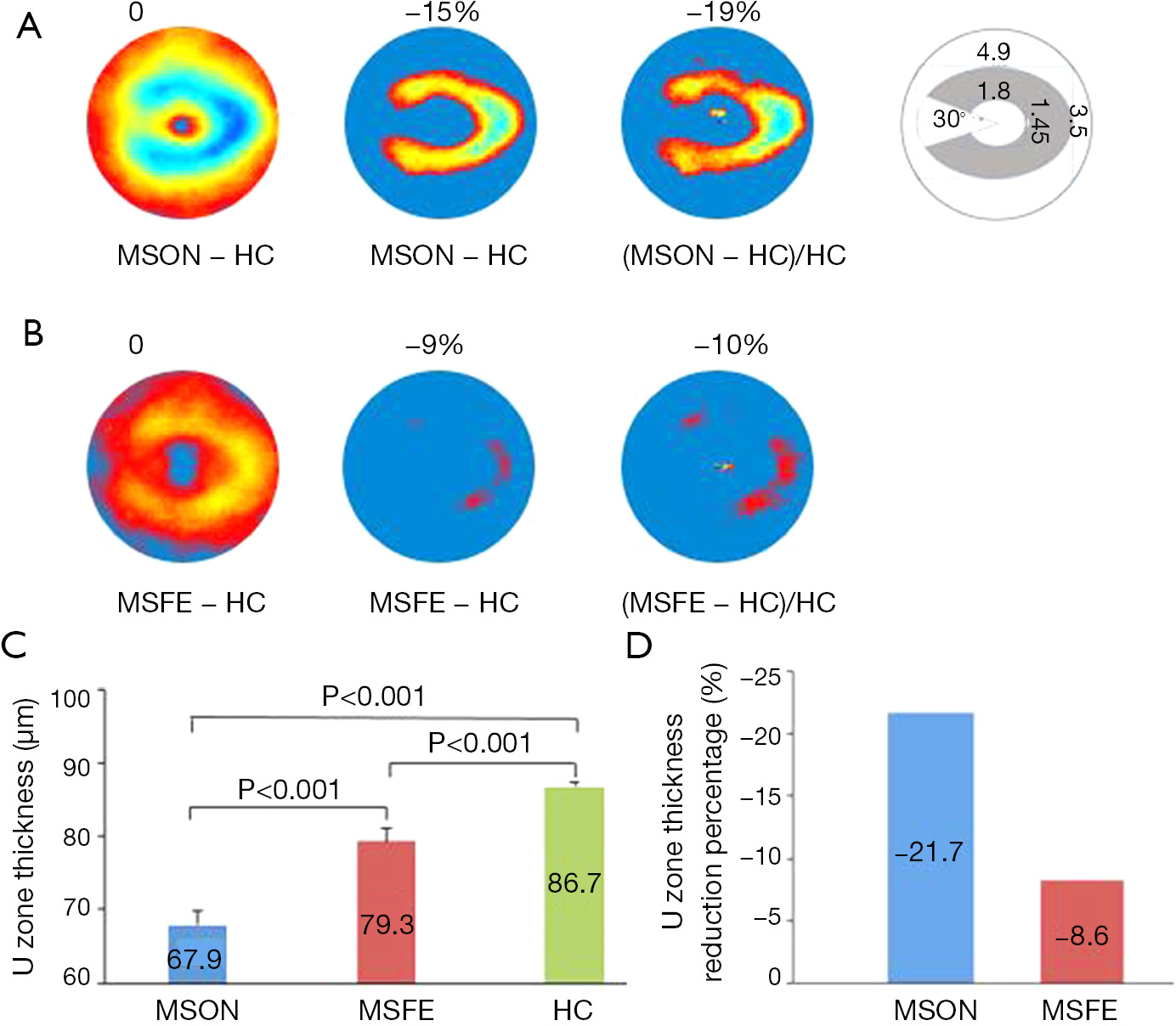
For the MS diagnosis, there is another challenge in that subclinical ON often occurs but is difficult to diagnose. Current imaging diagnostic criteria include lesions of the brain and spine but not the optic nerve. Clinical and subclinical ON are highly prevalent during the MS disease course (76-78), and image markers for ON are under development. A recent study suggested that inter-eye differences in pRNFL and GCIPL thicknesses can identify a history of unilateral ON, which may add the diagnosis of ON. However, inter-eye difference has only moderate differential power at 70% to identify ON from healthy control subjects (10,22,24). Visualization and quantitative analysis of the intraretinal layers may help define the most profound thinning zone in MS patients with ON eyes. Hu et al. used the same method used in the study by Shi et al. (14) to visualize the changes in thickness maps and reported a horseshoe-like thinning zone named the “U Zone” in macular GCIPL in MS patients with ON (Figure 5) (13). The GCIPL thickness alteration in the “U Zone” had the greatest differential power (AUROC =0.97) to identify MS patients with ON eyes from normal eyes. The intereye difference in the “U Zone” had a differential power at 0.85 to identify ON eyes from healthy control eyes (8). The discovery of the “M zone” and “U zone” indicates that detailed analysis of thickness maps may provide a better understanding of eye degeneration and could further improve the diagnostic power of MS-related ON. The established relationships of these focal thickness alterations and clinical manifestations demonstrate their value in the clinical management of neurodegenerative disorders.
The most common form of dementia in elderly people is AD (79). Neurodegeneration due to AD usually occurs decades before clinically detectable cognitive function decline (80). The transitional phase between normal cognition and AD is MCI. Patients with MCI can maintain their independent normal life, and ~15% MCI transfers to AD (80). Monitoring cerebral neurodegeneration may provide information on disease onset and progression. Brain MRI and positron emission tomography are the main tools but are expensive, sometimes invasive and time-consuming (81,82). The retina is an extension of the brain, and neurodegenerative progress in the retina may reflect neurodegenerative progress in the brain. OCT has been used for a long time to noninvasively measure the thickness of retinal neural layers, including RNFL and GCIPL, which are also used as biomarkers to monitor AD progress (31).
Recent meta-analysis studies revealed that thinning RNFL occurs in patients with MCI and/or AD (83,84). The Rotterdam Study found that thinner RNFL was associated with a higher risk of dementia in subsequent years (85). The thinning of RNFL was also related to worse MRI variables in the volume of hippocampus, global cingulum and posterior thalamic radiations (86). The GCIPL in patients with AD and MCI was also thinner compared with that of normal subjects (32,87,88). Using the Zeiss elliptical partition method, Cheung et al. found that the IT sector was the most affected region in patients with MCI but an inferior sector in patients with AD (32). Choi et al. used the Zeiss elliptical partition and drew a similar conclusion that the IT sector was thinner than other sectors in patients with MCI (87). Shao et al. used custom UHR-OCT and automated retinal segmentation software to visualize the topographic thickness maps of the intraretinal layers (15). More interestingly, by subtracting the GCIPL layer in cognitively normal subjects from that in MCI patients and AD patients, a focal thickness reduction was found in the superior outer region in patients with MCI and the superior inner region in AD using the ETDRS partition (15). This focal thickness reduction was more profound compared to any other sectors based on the Zeiss elliptical partition method. The findings are in agreement with previous histological studies that the peripheral retina, mainly containing parasol RGCs in the magnocellular pathway, is more involved in AD. Inferior visual field loss was also evident in patients with AD, and prominent Aβ deposition was found in the superior temporal quadrant in flat-mount retinas from patients with AD (89). However, whether the focal thinning in the superior quadrant was more related to disease onset and progression compared to average thickness reduction at the inferior quadrant needs to be further confirmed in future studies.
PD is a neurodegenerative disease that presents mainly with tremor, rigidity, akinesia and postural instability (90) due to selective dopamine neuron damage (91). Visual impairments such as decreased contrast sensitivity, visual acuity and impaired color vision often occur in patients with PD (92,93). A recent meta-analysis study showed thinning of pRNFL and GCIPL (94). Changes in the thickness of the intraretinal layers in PD may result from decreased dopamine (95) and accumulation of pathologic alpha-synuclein deposits in the retina (96).
Furthermore, the thinning of pRNFL mainly occurred in the inferior sector, followed by the superior sector (94). Sari et al. reported that the thicknesses of all sectors in the Zeiss elliptical partition of GCIPL in patients with PD were significantly lower than normal healthy controls (97). Bayhan et al. also found that average GCIPL, superior GCIPL, and inferior GCIPL were thinner in patients with PD compared with those in normal healthy subjects (98). Both studies found that the thickness of the inferior sector of GCIPL was correlated with disease duration and severity (97,98). Therefore, the inferior sector of GCIPL has the potential to be an imaging biomarker to monitor disease severity and duration. However, the alteration of the thickness map of GCIPL in patients with PD was not visualized in these previous studies.
In conclusion, we have summarized focal thickness changes of the intraretinal layers in some ocular and cerebral neurodegenerative disorders. These focal alterations in the intraretinal layers appear to provide better differential power and stronger correlations with clinical manifestations compared with those of the traditional partition method. Future longitudinal and large sample studies are needed.