Background: To investigate the effect of sirolimus (SRL) eye drops on acute alkali-burn-induced corneal neovascularization (CNV) and explore its possible mechanism.
Methods: A total of 57 male Sprague-Dawley rats weighing 160–180 g were randomly divided into four groups including a normal control group (NC group, n=12), an untreated alkali-burned model control group (MC group, n=15), a blank eye drop treatment group (BT group, n=15), and an SRL eye drop treatment group (ST group, n=15). Corneal inflammation and CNV were observed and scored under a slit-lamp microscope 3, 7, and 14 days after alkali exposure. Three rats were randomly sacrificed in each group before modeling and 3, 7, 14 days after modeling, and the corneas of right eyes were harvested for Western blotting to compare the expression levels of VEGFR2 and caspase-3.
Results: Corneal inflammation scoring showed that the corneal edema and conjunctival congestion were severe in the MC, BT, and ST groups 1 day after alkali exposure but were alleviated at day 3. The corneal transparency was significantly higher in the ST group than in the MC and BT groups at days 7 (F=9.77, P<0.05) and 14 (F=5.81, P<0.05). At day 1, the corneal limbal vascular network was markedly filled. SNV was obvious at days 3, 7, and 14. The new blood vessels were shorter and sparser in the ST group than in the MC and BT groups, and the CNV scores showed significant differences among these groups (day 3: F=8.60, P<0.05; day 7: F=11.40, P<0.05; and day 14: F=41.59, P<0.01). Western blotting showed that the expressions of VEGFR2 and caspase-3 were low before modeling and showed no significant difference among the different groups (F=0.52, P>0.05; F=0.98, P>0.05). The corneal expression of VEGFR2 became significantly higher in the MC and BT groups than in the ST group 3, 7, and 14 days after alkali exposure, and there were significant differences in relative gray-scale values among these groups (day 3: F=32.16, P<0.01; day 7: F=85.96, P<0.01; day 14: F=57.68, P<0.01). The increase in the corneal expression of caspase-3 was significantly larger in the ST group than in the MC and BT groups at days 3, 7, and 14, and there were significant differences in relative gray-scale values among groups (day 3: F=32.16, P<0.01; day 7: F=53.02, P<0.01; day 14: F=38.67, P<0.01).
Conclusions: SRL eye drops can alleviate acute alkali-burn-induced corneal inflammation and inhibit alkali-burn-induced CNV in rat models. It can reduce VEGFR2 expression and increase caspase-3 expression in the corneal tissue, which may contribute to the inhibition of alkali-burn-induced CNV.
The cornea is an important part of the refracting media of the eye. It is normally clear and transparent, and its vessel-free state is particularly important for maintaining the normal corneal function (1). When the cornea is stimulated by various pathogenic factors such as inflammation, hypoxia, trauma, and/or chemical burns, new capillaries can grow from the corneal peripheries into the center of the cornea. When the new blood vessels grow 1.0–2.0 mm into the limbs, they are defined as a pathological state and known as corneal neovascularization (CNV) (2). CNV can lead to visual impairment and even immune rejection after corneal transplantation. The currently available treatments for CNV mainly include laser treatment, photodynamic therapy, and medical treatments (e.g., immunosuppressive agents and monoclonal antibodies) (3). However, they are limited by poor effectiveness and/or high price. Therefore, it is necessary to find new drugs with low toxicity, good efficacy, and convenience.
Sirolimus (SRL), also known as rapamycin (RPM), is a novel macrolide antibiotic immunosuppressant that is 100-fold more active than cyclosporin A (CsA) but has milder toxicities than CsA and tacrolimus (FK506) (4). We had developed the SRL eye drops through prescription screening and technical development and had carried out a pharmacokinetic study in rabbit aqueous humor (5). The results showed that the SRL could quickly pass through the eye tissue after a single eye drop and reach a high concentration in the aqueous humor. In our current study, we used the Sprague-Dawley (SD) rats to establish alkali-burn-induced CNV models, in which the regulatory effect of SRL eye drops on the alkali-burn-induced CNV was observed, and its possible mechanism was explored with an attempt to assess whether SRL can be used as an ophthalmic drug in the prevention and treatment of CNV.
SRL eyedropswere provided by the Ophthalmic Preparation Laboratory of Zhongshan Ophthalmological Center, Sun Yat-sen University. The product was a white suspension liquid at a pH of 5–7, and the specification was 5 mL:5 mg. The drug was formulated as follows: after the suspending agent was dissolved by water for injection, SRL was added and then dispersed evenly at high speed. The other raw materials were dissolved by using water for injection and then added into the above suspension. After high-pressure homogenization, the drug solution was collected for further use. A blank solvent without SRL was used as a blank control eyedrop.
The 0.5% compound tropicamide eye drops and ofloxacin eye drops were purchased from Santen, Japan. The 10 g/L tetracaine eye drops were prepared by Zhongshan Ophthalmological Center. Mouse anti-VEGFR2 antibody, mouseanti-caspase-3 antibody, mouse anti-β-actin antibody, and horseradish peroxidase-labeled goat anti-mouse IgG were purchased from Abcam, UK.
A total of 57 male Sprague-Dawley rats (SPF grade) weighing 160–180 g were purchased from the Experimental Animal Center of Southern Medical University [Animal Certificate No. SCXK (Guangdong) 2016-0041]. They were randomly divided into four groups including the normal control group (NC group, n=12), the untreated alkali-burned model control group (MC group, n=15), the blank eye drop treatment group (BT group, n=15), and the SRL eye drop treatment group (ST group, n=15).
Animal experiments were performed in a manner consistent with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Animal Ethics Committee of the Zhongshan Ophthalmology Center. The animals were housed in the Experimental Animal Center of Zhongshan Ophthalmology Center of Sun Yat-sen University. They were maintained under controlled light schedule (12 hours light:12 hours dark) at room temperature (20–24 °C) and with constant humidity (55%). The animals had free access to food and water.
Mice were anesthetized with an intraperitoneal injection of 100 g/L chloral hydrate solution at a dose of 4 mL/kg. After routine iodophor disinfection, an eyelid opener was used for opening the mouse’s eye, followed by rinsing the right conjunctival sac with normal saline and instilling the eyes with 10 g/L tetracaine hydrochloride eye drops. A single-layer circular filter paper (3 mm in diameter) was prepared and immersed in a 1 mol/L of sodium hydroxide solution for 1 min. After the excess liquid was drained, the filter paper was quickly applied onto the surface of the center of the cornea for the 40 s and then rinsed with normal saline for 1 min. In the NC group, a circular filter paper that had been soaked in normal saline for 1 min was applied, and saline was used as the eye drop. In the MC group, saline was applied as an eye drop after modeling. In the BT group, blank eye drops were applied for instilling the eyes after modeling. In the ST group, SRL eyedrops were instilled after modeling. Ofloxacin eye drops were instilled into the right eyes in all groups (including NC group) after modeling to prevent infection. The eye drops were given at a dose of 20 μL four times a day at fixed time points for 14 consecutive days.
Corneal inflammation was observed under slit-lamp microscope 3, 7, and 14 days after alkali exposure. According to the scoring criteria proposed by Ueno et al. (6), the inflammatory response score was calculated based on indicators including the congestion of limbal vasculature, the corneal edema, and the healing of the corneal epithelium.
CNV was observed and scored under slit-lamp microscope 3, 7, and 14 days after alkali exposure in 6 mice randomly selected from each group. Neovascularization was scored on a scale of 0?4 (7), where 0= no vessels at the corneal limbus; 1= vessels within 2 mm of the corneal limbus; 2= vessels in the corneal peripheries, accounting for less than two quadrants; 3= vessels in the corneal peripheries, accounting for less than three quadrants; and 4= vessels in the entire cornea.
In each group, three animals were randomly selected before modeling and 3, 7, and 14 days after modeling and then euthanized with overdose chloral hydrate. The right eye was removed, and the cornea was isolated along the limbus. The cornea was then quickly placed in liquid nitrogen for cryopreservation. Before the detection, the sample was ground in an ice bath. Then, 1.5 mL of pre-cooled radio immunoprecipitation assay buffer (RIPA) was used to lyse the tissue and extract the proteins. After the protease inhibitor was added, BCA (Bradford) protein quantification assay was performed. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the target proteins were transferred onto a PVDF membrane by semi-dry blotting. After having been blocked with 5% skimmed milk powder for 1h, the mouse anti-VEGFR2 (1:500), caspase-3 (1:500), and β-actin (1:1 000) monoclonal antibodies were added overnight at 4 °C. Horseradish peroxidase-labeled goat anti-mouse IgG secondary antibody (1:5,000) was added and then incubated at room temperature for 1h. Protein signals were detected by enhanced chemiluminescence (ECL). Calculation of the relative gray values was based on the following formula: the relative gray-scale value of the target protein = gray value of the target protein band/gray value of the β-actin band ×100%.
Statistical analysis was performed using SPSS 25.0 software package. Measurement data are expressed as mean ± standard deviation (mean ± SD). Inter-group differences were analyzed by one-way ANOVA. Bonferroni Test was applied for multiple comparisons after the test for homogeneity of variances. A P value of <0.05 was considered statistically significant.
The corneas were clear and transparent, without any edema, congestion, or other inflammatory reactions in the NC group at each time point and in the MC, BT, and ST groups before modeling. However, the corneal edema and conjunctival congestion were severe in the MC, BT, and ST groups 1 day after alkali exposure. At day 3, the corneal edema and conjunctival hyperemia were alleviated in these three groups, and the corneal inflammation score showed no significant difference among these groups (F=0.60, P=0.56). At day 7, the corneal transparency increased in the ST group, and the corneal inflammation scores became significantly different among the three groups (F=9.77, P<0.05). At day 14, the corneal transparency was further increased in the ST group compared with the MC and BT groups, and the difference was statistically significant among these three groups (F=5.81, P<0.05). The corneal inflammation scores in each group are shown in Table 1. The corneal inflammation scores of the ST group were significantly different from those of the MC group and BT group 7 and 14 days after alkali exposure (all P<0.05), whereas there was no significant difference in corneal inflammation scores between the MC group and BT group. The corneal transparency increased gradually in the MC, BT, and ST groups over time and the inflammation gradually decreased, especially in the ST group. Therefore, SRL eye drops could effectively alleviate corneal inflammation induced by alkali burns.
| Group | The score of the corneal inflammatory response | ||
|---|---|---|---|
| 3 d | 7 d | 14 d | |
| MC | 6.07±0.52 | 4.83±0.75 | 3.50±0.55 |
| BT | 6.50±0.55 | 5.17±0.75 | 3.33±0.82 |
| ST | 6.33±0.52 | 3.50±0.55*# | 2.33±0.55*# |
*, P<0.05, compared with MC group; #, P<0.05, compared with BT group.
The corneas were clear, transparent, and without any new vessels in the NC group at each time point and in the MC, BT, and ST groups before modeling. After 1 day of alkali exposure, edema and turbidity were observed at the center of the cornea, and the corneal limbal vascular network was markedly filled. At day 3, the new blood vessels were short and sparse in the ST group; in contrast, the new vessels were denser and larger in number and grew into the limbus. The CNV scores showed significant differences among these groups (F=8.60, P<0.05). At day 7, the new vessels in the ST group were thin and sparse; they entered the cornea but did not reach the burn area. In contrast, the new vessels in the MC group and the BT group were thicker and denser, and their growth was deeper and approached the edge of the center of the cornea. The CNV scores showed significant differences among these groups (F=11.40, P<0.05). At day 14, new vessels were seen in almost entire corneas in the MC group and BT group, while the neovascularization in the ST group did not progress further. The difference among these groups was statistically significant (F=41.59, P<0.01). The results of neovascularization scoring are shown in Table 2. The neovascularization scores of the ST group were significantly different from those of the MC group and BT group 3, 7, and 14 days after alkali exposure (all P<0.05) but did not significantly differ between the MC group and BT group. As shown in Table 2, CNV progressed over time in the MC and BT groups; in contrast, the ST group had the fewest newly formed blood vessels. Therefore, SRL eye drops could effectively inhibit alkali-burn-induced CNV in rat models.
| Group | CNV score | ||
|---|---|---|---|
| 3 d | 7 d | 14 d | |
| MC | 1.33±0.52 | 2.67±0.55 | 3.50±0.55 |
| BT | 1.50±0.55 | 2.50±0.55 | 3.33±0.52 |
| ST | 0.33±0.52*# | 1.33±0.52*# | 1.17±0.41**## |
*, P<0.05; **, P<0.01, compared with the MC group; #, P<0.05, ##, P<0.01, compared with BT group.
Western blotting showed that SRL eyedrops could regulate the expressions of VEGFR2 and caspase-3 in rat corneas. As shown in Figure 1, the expressions of VEGFR2 were low before modeling and showed no significant difference among different groups (F=0.52, P>0.05). At day 3, the corneal expression of VEGFR2 significantly increased in the MC, BT, and ST groups, and there was significant difference in the relative gray-scale values among these three groups (F=32.16, P<0.01); in particular, the increase in VEGFR2 expression was significantly higher in the MC and BT groups than in the ST group (P<0.05). At day 7, the corneal VEGFR2 expression levels reached their peaks in MC and BT groups and there was significant difference in relative gray-scale values among these three groups (F=85.96, P<0.01); notably, the VEGFR2 expression at day 7 was not significantly different from that at day 3 in the ST group (P>0.05). At day 14, the relative gray-scale values were still significantly different among these groups (F=57.68, P<0.01); while the expression of VEGFR2 decreased compared with that at day 7 in MC, BT, and ST groups, it was still significantly higher in the MC and BT groups than in the NC group (P<0.01); only the VEGFR2 expression in the ST group decreased to a level comparable with the NC group (P>0.05).
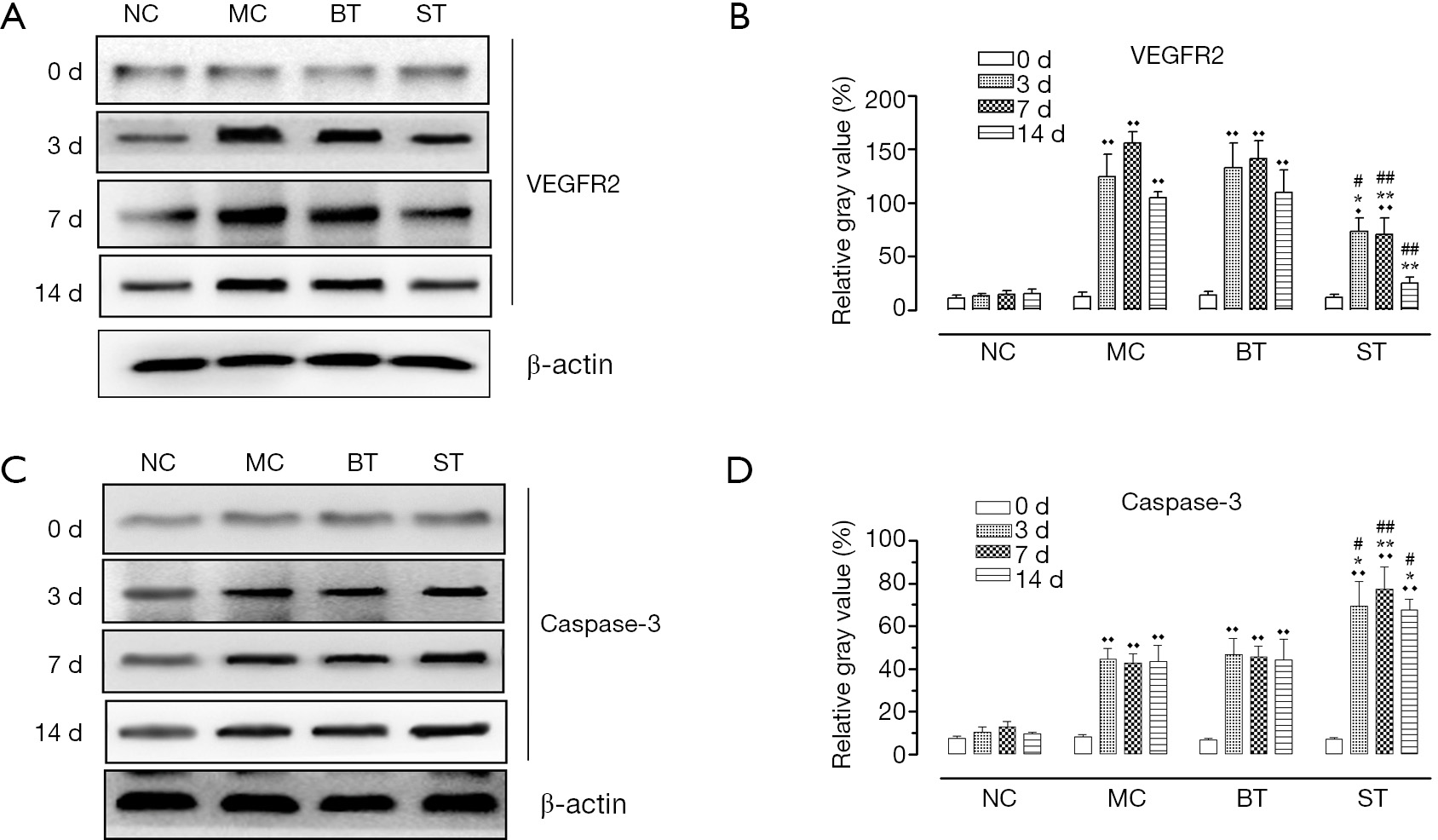
The expression of caspase-3 was also low in normal corneas, and the differences among different groups were not statistically significant (F=0.98, P>0.05). At day 3, the corneal expression of caspase-3 significantly increased in the MC, BT, and ST groups, and there was significant difference in relative gray-scale values among these three groups (F=32.16, P<0.01); unlike VEGFR2, the increase in caspase-3 expression was higher in the ST than in the MC and BT groups (P<0.05). At days 7 and 14, the relative gray-scale values significantly differed among these three groups (F=53.02, F=38.67; P<0.01); however, the corneal expression of caspase-3 showed no significant difference when compared with the level at day 3 in the MC, BT, and ST groups.
Corneal inflammation caused by alkali burns is a major cause of CNV. Inflammatory cells infiltrating corneal tissue can release a variety of cytokines, further aggravating tissue necrosis. Once the corneal pro-angiogenic factors and anti-angiogenic factors become imbalanced, the blood vessels will emanate along the limbal capillaries and venules and grow towards the center of the cornea, causing the development of CNV (1,2). Corneal opacity due to neovascularization not only seriously affects vision but also destroys the characteristics of corneal immune privilege, leading to the severe damage of the structure and function of the eyes and resulting in corneal edema, lipid deposition, and corneal scar formation (8). At present, hormonal anti-inflammatory drugs remain the most simple and effective method in treating corneal alkali burns. However, hormones may accelerate corneal lysis/perforation and induce glaucoma (3). Therefore, it is much necessary to research and develop new drugs with lower toxicity, better efficacy, and more convenience.
As an immunosuppressant, SRL can suppress the proliferative response of T lymphocytes, inhibit the G1-to-S phase transition, block the binding of interleukin-2 (IL-2) to its receptor, and thus prevent Tc and Td cells from becoming sensitized T lymphocytes with an immune response. According to Dantal et al. (9), SRL could effectively lower the incidence of skin cancer after renal transplantation; compared with those taking CsA or FK506, patients taking SRL had a 50% lower risk of developing new squamous cell carcinoma. They speculated that SRL had a special anti-tumor activity, which might be achieved by inhibiting angiogenesis and cell proliferation. As shown in our current study, SRL eye drops could effectively alleviate the alkali-burn-induced inflammatory reaction in rat corneas and significantly inhibit CNV induced by alkali burns.
Vascular endothelial growth factor (VEGF), which is closely related to angiogenesis and vascular permeability, has attracted much attention in studies on anti-angiogenesis. VEGF acts by binding to downstream VEGF receptors. The VEGF receptors that have been discovered and cloned mainly include VEGFR1 (Flt-1), VEGFR2 (Flk/KDR), and VEGFR3 (Flt-3), of which VEGFR2 is the most important functional receptor in the VEGF pathway (10,11). In addition, caspase-3 is an important executor of cell apoptosis (12,13). Therefore, our current study focused on the expression changes of these two protein molecules during the corneal alkali burn in rats treated with SRL eye drops. The results showed that SRL eye drops could inhibit the expression of VEGFR2 and promote the expression of caspase-3, which may be the mechanisms by which the drug inhibits the alkali-burn-induced CNV.
The topical use of SRL eye drops in the eyes can effectively reduce the side effects of systemic medications and also avoid infections and other complications that may be caused by ocular injections. As a promising new drug, SRL eye drops deserve further investigation and application.