Abstract: Retinopathy of prematurity (ROP) is an emerging cause of childhood blindness in the developing countries. The low and middle-income countries are facing common challenges in the midst of the ‘third epidemic’ of ROP. Improvement in neonatal care facilities has increased survival of preterm babies. Lack of awareness and non-uniform standards of care in the ever-increasing number of neonatal intensive care units (NICUs) and special newborn care units (SNCUs) has resulted in this surge of ROP. Apart from low birth weight and the degree of prematurity, use of unblended supplemental oxygen, sepsis, anemia and blood transfusion are important risk factors associated with ROP in developing countries. Atypical forms of aggressive posterior ROP (APROP) are seen in heavier birth weight babies in the developing countries. Prevention of ROP by good quality neonatal care, timely diagnosis by mandatory ROP screening in NICUs and training manpower for laser treatment of ROP requires close collaboration between the neonatologists, ophthalmologists and the policy makers. Team approach and inter-disciplinary co-ordination are keys in a nation’s drive to fight this preventable cause of blindness.
Retinopathy of prematurity (ROP) is a proliferative retinopathy affecting the premature retina. The spectrum of ROP varies from mild self-limiting disease to total retinal detachment and irreversible blindness. Over 50,000 children are blind from ROP (1) across the globe, despite the availability of a highly effective therapy in the form of laser photocoagulation. ROP is a major cause of potentially preventable childhood blindness in the industrialized west and is an emerging cause of childhood blindness in developing countries like India, Latin America, Eastern Europe, and China (1-3).
ROP occurs worldwide; however, its incidence varies widely across different continents. Gilbert et al. (1) reported that the variability of the incidence rates across different regions could be correlated with the infant mortality rates (IMRs) of the country. These indirectly reflect the quality and accessibility of health care facilities and the level of socioeconomic development. In countries with high infant mortality rates (>60 per 1,000 live births) such as Sub-Saharan Africa, ROP occurrence is rare due to poor survival of premature babies. In the developed countries (IMR <9 per 1,000 live births), the incidence of severe ROP has been reported to be less than 20% (4-8).
However, it is the low and middle income countries (IMRs ranging from 9 to 60 per 1,000 live births) which are at present facing an “epidemic” of ROP blindness often referred to as the “third epidemic” of ROP. These are the countries where neonatal care facilities are improving, leading to increasing survival of premature babies. Inadequate neonatal support, non-uniform neonatal care practices, lack of awareness about ROP, absence of ROP screening programs in the neonatal intensive care units (NICUs) and lack of trained ophthalmologists to diagnose and treat ROP are the primary reasons behind the surge of ROP in developing countries. This epidemic of ROP is somewhat similar to the first epidemic of ROP seen in the west in the 1940s and 50s, which was ascribed to the unmonitored use of oxygen to treat respiratory distress at birth.
In India, ROP has been reported to occur in 21.7–51.9% of low birth weight infants (9-14). Table 1 compares the various studies reporting the incidence of ROP from India and some other developing countries. Most of the studies report mean birth weights above 1250 g and incidence of severe ROP ranging from 5.0–44.9%. ROP has also been reported as an important cause of childhood blindness in several Latin American countries. Blind school surveys (3) revealed ROP as the cause of blindness in 38.6% (Cuba), 33.3% (Paraguay), 17.6% (Chile), 14.1% (Ecuador), 10.6% (Colombia) and 4.1% (Guatemala) of children surveyed in different countries in Latin America. Reported rates of treatable ROP varied from 19.1% in Lima, Peru (17) to 5.9% in Southern Brazil (15).
| Study | Period | Inclusion criteria | Mean birth weight (g) | Mean gestational age (wks) | ROP incidence | Incidence of treatable/sight threatening ROP |
|---|---|---|---|---|---|---|
|
Charan |
1995 | BW ≤1,700 g | 1,382.8 | 32.5 | 47.2% | 12.7% |
|
Gopal L |
1995 | BW ≤2,000 g | 1,477.6 | 32.4 | 38% | 16.0% |
|
Jalali |
2006 | BW <2,000 g; GA <36 wks | 1,254.5 | 29.6 | – | 11% |
|
Chaudhari |
2009 | BW<1,500 g; GA ≤32 wks | 1,306 | 31.4 | 22.3% | 33.3% |
|
Fortes Filho |
2009 | BW ≤1,500 g; GA ≤32 wks | 1,205.5 | 30.3 | 25.5% | 5.8% |
|
Hungi |
2012 | BW ≤2,000 g; GA <34 wks | 1,555.9 | 32.2 | 41.5% | 10.2% |
|
Xu |
2013 | BW ≤2,000 g; GA ≤34 wks | 1,425 | 29.9 | 17.8% | 6.8% |
ROP, retinopathy of prematurity; BW, birth weight; GA, gestational age; wks, weeks.
Several studies have highlighted the need for wider and more inclusive screening criteria in middle-income economies compared to the west. In India, Jalali et al. (11) in their study showed that 13.3% of babies exceeded the United Kingdom and United States screening criteria. Hungi et al. (13) in a recent study reported 57.6% of the babies were heavier and older than the American screening cut-off. Of these, 36.8% had some stage of ROP and 8% required treatment. Vinekar et al. (18) reported that 17.7% babies with severe ROP would be missed using the American guidelines and 22.6% using the British screening guidelines for screening. Gilbert et al. (19) reported that 13% of infants from several middle and low-income countries would not have been screened for ROP if the UK screening criteria were followed. These reports strengthened the evidence and led to modification of screening criterion in India for ROP. Screening guidelines that are being followed in India since 2010 include:
The first screening examination for ROP is recommended by ‘day 30’ of life, irrespective of the gestational age. Infants <28 weeks or <1,200 g should be screened early at 2–3 weeks of age to enable early identification of aggressive posterior ROP (APROP).
There is a consensus now on including ROP a part of universal eye screening in babies with birth weights up to 2,000 gm and/or gestational age up to 35 weeks or Infants with an unstable clinical course who are at high risk (as determined by the neonatologist or paediatrician) under a government run national program known as Rashtriya Bal Swasthya Karyakram (RBSK).
The retina of a preterm infant is incompletely vascularized at birth. ROP occurs if the postnatal environment does not match the in utero environment that supported the retinal vascular and neural development. Amongst the most consistent risk factors for ROP is the degree of prematurity itself. The lower the birth weight and the gestational age the higher is the risk for ROP. These are probably now the major risk factors in regions where neonatal care is of high quality such as in the industrialized west. A number of other postnatal factors may contribute to the development of ROP. These risk factors are important causes of ROP in the developing countries and include use of supplemental oxygen (20-22), intraventricular hemorrhage (23), apnea (24), mechanical ventilation (24), sepsis, surfactant therapy (25), anemia (26), administration of blood products as well as double volume exchange transfusions (27).
APROP in heavier and more mature babies has been reported with the use of unblended oxygen in India (28). Fluorescein angiography done in these eyes showed massive capillary dropout similar to the vaso-obliteration seen in experimental animal models of ROP produced by oxygen (29). Low postnatal weight gain proportion (i.e., weight gain less than 50% of the birth weight) by 6 weeks of life is being considered now as an independent risk factor for ROP as well as a predictor for severe ROP (30).
The immature retina is susceptible not only to the higher than in-utero oxygen levels but also to variability in the oxygen tensions and lack of nutrients and growth factors normally provided in utero resulting in cessation of vessel growth. Neonatologists as well as nurses can play a major preventive role by implementing evidence based as well as cost-effective practices in the NICU to reduce some of the risk factors such as following strict pre-defined oxygen saturation targets, implementation of asepsis, hand hygiene routines, encouraging breast feeding, kangaroo mother care and restrictive blood transfusion policies.
The clinical features of ROP include a demarcation line at the junction of vascular and avascular retina, development of the “ridge” tissue, extraretinal fibrovascular proliferation growing from the retinal surface into the vitreous, traction retinal detachment, total retinal detachment, leucoria, falciform fold and retinal as well as vitreous hemorrhage. Generally, ROP passes through five stages (I–V), which have been clearly defined by the International Classification of ROP (ICROP) (31) and are universally followed. Depending upon the location of the retinopathy, ROP has been divided into three zones. ROP typically progresses stage wise/step-wise until treatment stage is reached or total retinal detachment occurs which leads to irreversible blindness.
A more severe and aggressive form of ROP, known as APROP has been described in the recent ICROP classification (31) which has the propensity to progress directly to stage V without passing through the intervening stages. Developing countries are particularly facing a surge of APROP and are thus faced with a challenge in the early management of such potentially blinding cases. Table 2 compares the spectrum of APROP reported from various studies in India and other industrialized countries. This form of ROP occurs in babies with extremely low birth and gestational ages in the west (32,34). On the contrary in the developing countries, APROP is occurring in babies with much higher birth weight (Figure 1) as well as gestational age (28,33,35,36). This form of APROP may progress rapidly forming an indication for doing screening earlier than “Day 30 of life” i.e., within 2–3 weeks after birth. APROP in heavier babies (36) has been reported to occur most commonly in posterior zone II with more mature vasculature in the posterior retina as compared to the poorly developed posterior vasculature in zone I APROP. In addition, vessels extend for a considerable distance into the nasal retina forming large loops (Figure 2) enclosing the avascular retina.
| Author | Year of publication | Country | Mean birth weight (g) | Mean gestational age (wks) |
|---|---|---|---|---|
|
Azuma |
2006 | Japan | 773 | 25 |
|
Sanghi |
2009 | India | 1,259 | 29.75 |
|
Drenser |
2010 | USA | 627 | 24.3 |
|
Jalali |
2011 | India | 1,228 | 29.63 |
|
Shah |
2012 | India | 1,572 | 31.7 |
|
Sanghi |
2014 | India | 1,791 | 30.7 |
ROP, retinopathy of prematurity; wks, weeks.
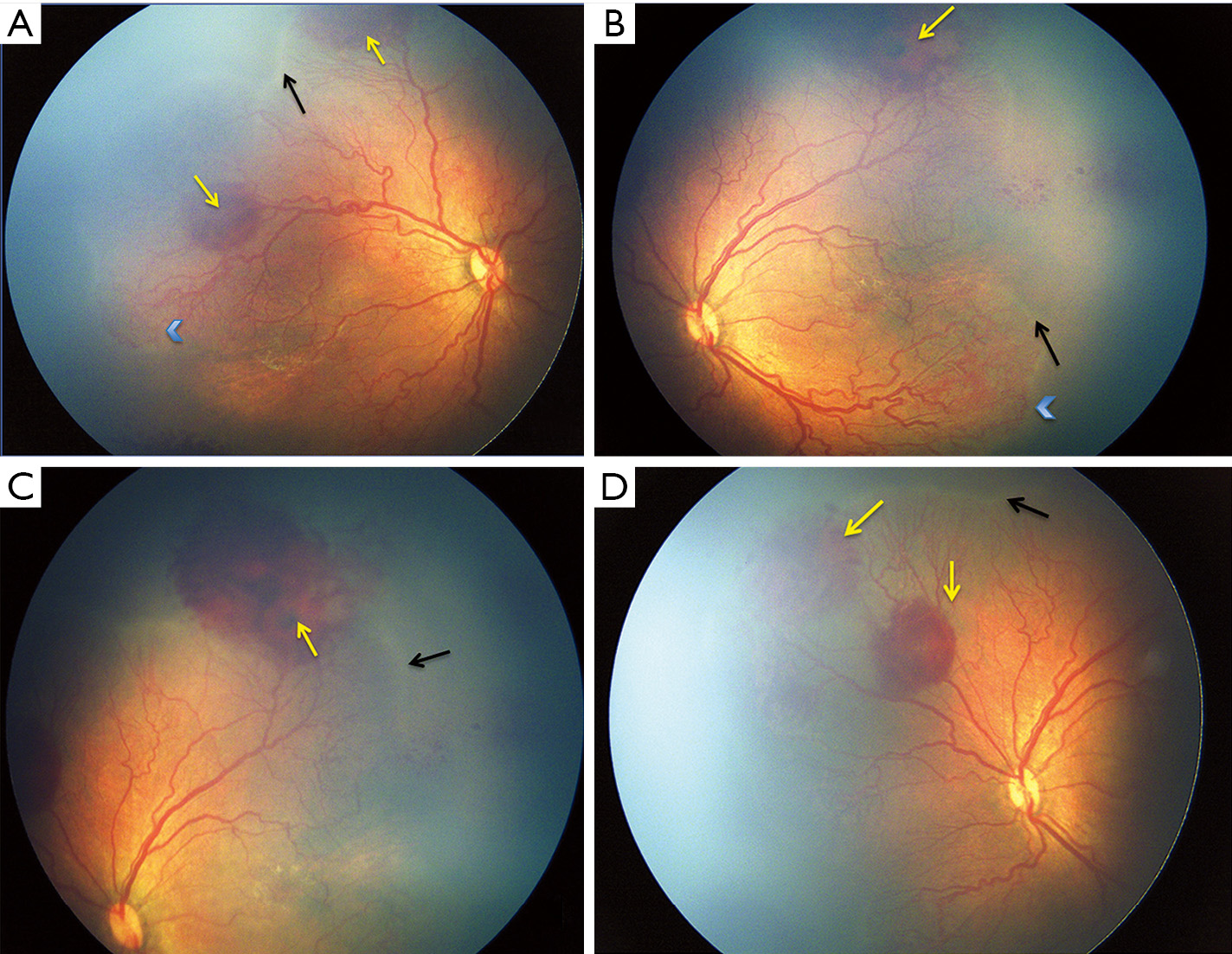
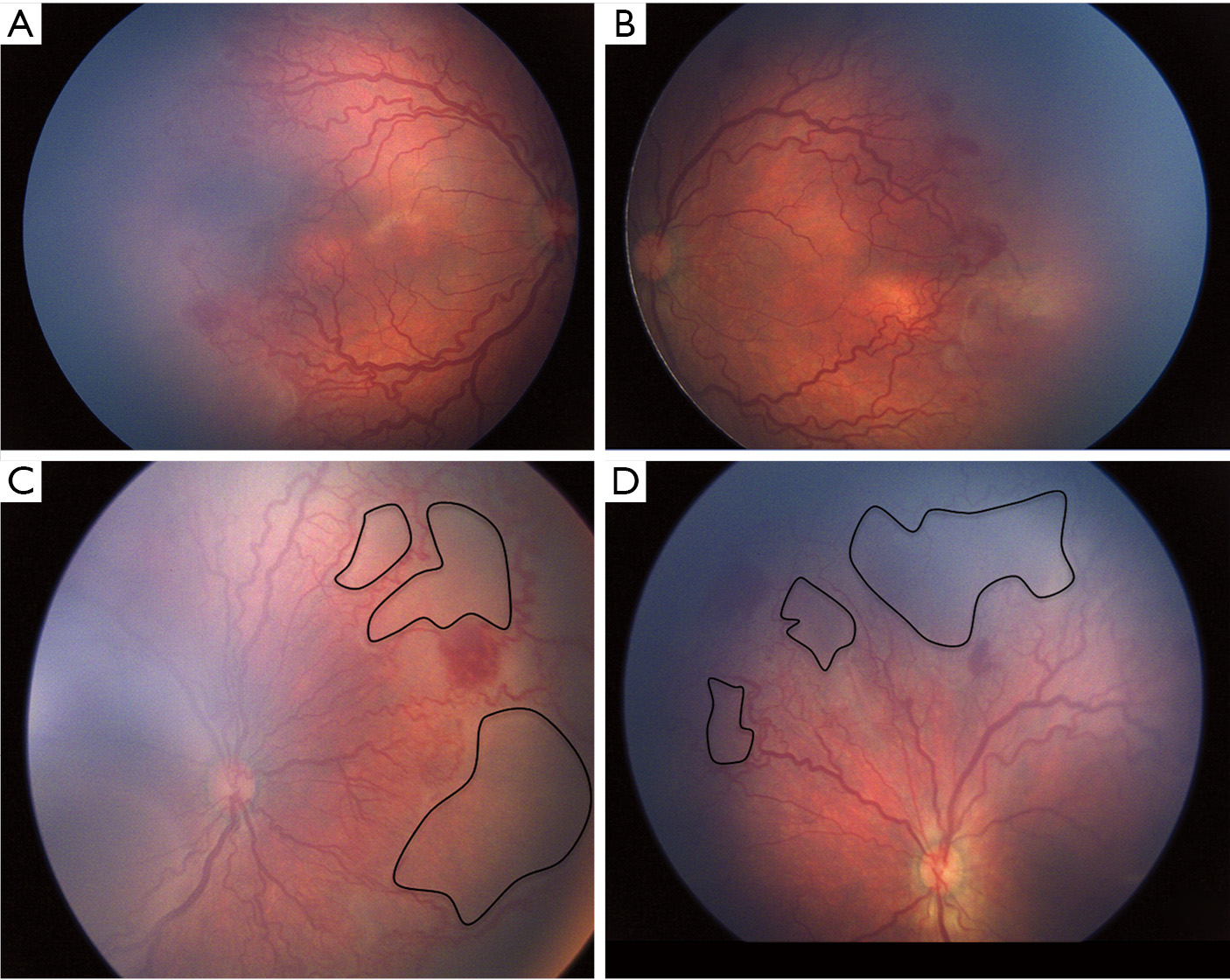
An atypical form of APROP termed as “Hybrid ROP” (Figure 1) has been reported from India, which is difficult to classify as per the current ICROP classification as it has features of both APROP as well as formation of “ridge” seen in staged ROP or mat like epiretinal proliferation on the vascularized posterior retina (36).
ROP treatment may be performed using either a diode laser or a 532-nm green laser whichever is available with equal efficacy (37). Early Treatment for Retinopathy of Prematurity (ETROP) study (38) recommendations are followed i.e., Zone I any stage with plus disease or Zone I, stage 3 without plus disease or Zone 2, stage 2 or 3 with plus disease. In addition to the ETROP study guidelines APROP warrants early treatment within 48–72 hours.
As has been highlighted earlier, the presentation as well as severity of ROP varies across different geographic regions in terms of the infants “at-risk” for blinding ROP. The screening guidelines followed in India for e.g., have been modified to maximize coverage and minimize the chance of missing severe ROP. Whereas timely diagnosis and treatment alone can provide excellent visual outcome in most cases, the biggest challenge faced by developing countries today is in fact the lack of timely screening. Most level 2 and 3 NICUs have no ROP screening programs and are hence not screening for ROP. This is coupled with highly variable standards of oxygen use and other neonatal care practices. ROP screening lagged behind the surge of the NICUs and Special Newborn Care Units (SNCUs) because of the lack of foresight that these would be potential hotbeds of blindness in the future. Lack of awareness amongst the planning and executing agencies, neonatal care teams as well as non-availability of trained and interested ophthalmologists is responsible for this. Countries with low ROP incidence right now (e.g., Sub-Saharan Africa) should take a cue from the experience of the middle income developing countries and plan their neonatal care expansion programs incorporating strict ROP screening protocols. Seeing a baby with stage V ROP in both eyes is the worst nightmare for any ROP expert, yet majority of them have to see more and more babies with stage V ROP from new geographic regions practically every week. It is extremely difficult to convince and counsel the family that the window of opportunity has been lost and the visual prognosis is poor at this stage. This has led to increasing incidences of medicolegal litigation in cases of ROP related blindness. In one report from a tertiary care referral centre (39) in India, 86.4% of infants who presented with stage V ROP had never been screened for ROP. Nearly 74.2% were brought by the parents (i.e., self-referred) when they noticed that child is not seeing. Pediatricians referred none of the infants for screening and 25.8% were referred by an ophthalmologist. Wide-field retinal imaging using Ret-Cam by trained technicians has been shown to be highly effective in the KIDROP program, a model program for developing countries (40). This model of using trained technicians for documenting disease and decision-making regarding treatment and referral had 95.7% sensitivity, 93.2% specificity and a positive predictive value of 81.5% compared to ROP experts. This may be the way forward where trained ophthalmologists are not available.
A close collaboration between neonatologists, ophthalmologists, healthcare workers, parents as well as policy makers is required to tackle the emerging problem of ROP blindness. There is a need for enforcement of strict laws regarding screening services for ROP in all the facilities where premature/high-risk neonates are being managed. Primary prevention through improvement of neonatal care and secondary prevention through case detection and treatment need to be focused upon. This should include provision of the necessary equipment and personnel trained in ROP detection and appropriate referral services. The ultimate aim is to reduce the incidence as well as the severity of ROP, detect and treat cases optimally so that these premature babies get an opportunity to see.