Refractive error is one of the most common ocular disorders worldwide, and its undercorrection is the leading cause of visual impairment (1,2). Refractive error, particularly undercorrection of myopia can also affect the performance at school, reduce employability and productivity, and may generally impair quality of life (2). Myopic individuals, particularly highly myopic persons, are susceptible to other ocular abnormalities and diseases, namely retinal detachment, myopic maculopathy and glaucomatous optic neuropathy (2,3). Despite the importance of myopia, only relatively few school-based studies on the prevalence or incidence of myopia have been carried out in China (4-8). Furthermore, previous studies were mainly performed in large cities in the east of mainland China, such as in Beijing and in Guangzhou. There is a paucity of data on the epidemiology of refractive errors in school children in the vast western parts of China, where, as compared with East Chinese metropolitan regions, education intensity, socio-economic conditions and climate are different. We therefore conducted this cohort study. The main purpose of the current study was to examine the prevalence and incidence of myopia and their associations in West China.
In addition to refractive error, the study also aims to investigate normative data of the school students, such as the intraocular pressure, the choroidal thickness and the retinal nerve fiber layer thickness and their associations, since previous studies on these issues were mainly involved adults or hospital-based studies with limited sample size and potential selection bias.
This review summarizes the major findings gathered so far from the Gobi Desert Children Eye Study.
The study methods have been described previously (9-12). The Gobi Desert Children Eye Study was a school-based cohort study, which was performed in the oasis city of Ejina in the Gobi Desert. The Ethics Board of the Affiliated Hospital of Inner Mongolia Medical University Hohhot and the local Administration of the Education and School Board of Ejina approved the study (Ethic ID YKD2018220) and informed written consent was obtained from the parents or guardians of all children.
As study region we chose a city in an oasis in the middle of the Gobi Desert in Inner Mongolia. This city of Ejina has the advantage that due to its isolated location, the exchange of the population with other regions is limited, and that the population is relatively stable. Ejina is located in the most western part of the Chinese province of Inner Mongolia and is characterized by extremely arid conditions. The study region belongs to the north temperature climate zone with a mean annual precipitation of approximately 40 mm. Average minimum winter temperatures are close to ?40 °C, while summer time temperatures can reach 50 °C.
The baseline study was carried out in 2013, which included all three schools in Ejina. Ejina has a total population of 18,030 inhabitants (including 11,301 Han Chinese, 6,209 Mongols and 520 individuals from other minorities). All children from Ejina attend one of the three schools. The three schools in Ejina are Ejina primary school (911 students), Ejina middle school (765 students), and Minority school (235 students) included altogether 1,911 children.
Three years later, the senior high school students graduated from Ejina middle school and left Ejina for college or work. All the other students who participated the baseline study and stayed in Ejina schools were invited for a repeated study in 2016.
All the participated students underwent comprehensive systemic and ophthalmological examinations and their parents were interviewed using a standardized questionnaire including questions on the profession, level of education, income, and ethnic background of both parents; the birth weight, birth age, and type of birth of the children; and whether oxygen was supplied after birth.
The non-ophthalmological systemic examinations included measurement of body height (using a stadiometer) and body weight, heart rate and blood pressure [using an automatic blood pressure monitor (YE655A, YUYUE, Jiangsu, China)]. The body mass index was calculated as the ratio of body weight (expressed in kg) divided by the square of body height (expressed in m).
The ophthalmological examinations included best corrected visual acuity, slit lamp-based examination of the anterior ocular segment by an ophthalmologist, intraocular pressure (IOP), fundus photography, spectral domain OCT, assessment of ocular motility, binocularity and presence of strabismus.
Visual acuity was measured using a LogMAR chart at 4-meter distance. IOP was measured by a non-contact tonometer (Canon TX-F Full-Auto Tonometer, Canon Co., Tokyo, Japan). Pre-and post-cycloplegic refractive errors were measured using an auto-refractor (ARK-900, NIDEK, Tokyo, Japan). For cycloplegic refraction, one drop of topical 1.0% cyclopentolate (Alcon, Ft. Worth, USA) was administered to each eye twice with a 5-minute interval and a third drop was administered 15 minutes after the second drop if the pupil size was less than 6 mm or if the pupillary light reflex was still present. Each eye was measured at least 3 times. The spherical equivalent (SE) of the refractive error was defined as the spherical value of refractive error plus one half of the cylindrical value. Myopia was defined as SE of more myopic than ?0.5 diopter (D). High myopia was defined as SE of more myopic than ?6.0 D. Hyperopia was defined as SE of more hyperopic than +0.5 D or +1.0 D, respectively, in the worse eye. The eye with higher absolute value of the refractive error was taken as the worse eye. Glasses worn by the parents were measured as an estimate of their refractive errors. The parents were not refracted.
After medical mydriasis, ophthalmoscopy was carried out for examination of the fundus. Spectral domain OCT (Spectralis, wavelength: 870 nm; Heidelberg Engineering Co., Heidelberg, Germany) with enhanced depth imaging (EDI) modality was performed after pupil dilation. The horizontal section running through the center of the fovea was selected for measurement of choroidal thickness, which was defined as the vertical distance from the hyper reflective line of the Bruch’s membrane to the hyper reflective line of the inner surface of the sclera. The measurement was performed using the built-in software. The thickness was measured at five points for each eye: subfoveal, 1,000 and 2,500 μm nasal to the fovea, and 1,000 and 2,500 μm temporal to the fovea.
Statistical analysis was performed using a commercially available statistical software package (SPSS for Windows, version 21.0, IBM-SPSS, Chicago, IL, USA). Descriptive statistics included mean, standard deviation (SD), median, range, and percentages were presented where appropriate. The normal distribution of parameters was tested by the Kolmogorov-Smirnov test. In the case of not normally distributed parameters, the Mann-Whitney test was applied to examine the statistical significance of difference between un-paired groups. The Chi-square test was used to compare proportions. Prevalence estimates of refractive errors using different definitions were calculated based on pre- and post-cycloplegic refraction data. Difference in pre- and post-cycloplegic refraction data was calculated. In univariate analysis we analyzed associations between the presence of myopia and other ocular and systemic parameters. We then performed a multivariate binary regression analysis, with the presence of myopia as dependent variable and all those parameters which were significantly associated with the presence of high myopia in the univariate analysis as independent variables. Linear regression analysis was performed to analyze the associations of intraocular pressure or choroidal thickness. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated. All P values were 2-sided and were considered statistically significant when the values were less than 0.05.
Out of 1,911 children who were primarily eligible for the study 346 refused the examination, so the baseline study eventually included 1,565 (81.9%) children [801 (51.2%) boys] with a mean age of 11.9±3.5 years (median: 11.7 years; range: 6 to 21 years). In terms of ethnicity, 1,264 fathers (80.8%) of the students were Han, and 282 (18%), 14 (0.9%), 4 (0.3%), and 1 (0.1%) were Mongolian, Hui, Man, and Erwenke, respectively. Correspondingly, 1,209 (77.3%), 335 (21.4%), 14 (0.9%), 3 (0.2%), 3 (0.2%), and 1 (0.1%) of the mothers were Han, Mongolian, Hui, Man, Tibetan, and Tujia, respectively. If either of the parents of a student was a member of a minority group, the student was considered to belong to minority group. There were 1,160 Han students and 405 students from minority groups (Table 1).
| Age (years) | n (%) | Gender, n (%) | Ethnicity, n (%) | |||
|---|---|---|---|---|---|---|
| Boys | Girls | Han | Mongolian and other ethnicities | |||
| 6 | 1 (0.1) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) | |
| 7 | 110 (7.0) | 50 (45.5) | 60 (54.5) | 71 (64.5) | 39 (35.5) | |
| 8 | 149 (9.5) | 73 (49.0) | 76 (51.0) | 104 (69.8) | 45 (30.2) | |
| 9 | 147 (9.4) | 70 (47.6) | 77 (52.4) | 100 (68.0) | 47 (32.0) | |
| 10 | 153 (9.8) | 92 (60.1) | 61 (39.9) | 127 (83.0) | 26 (17.0) | |
| 11 | 118 (7.5) | 58 (49.2) | 60 (50.8) | 91 (77.1) | 27 (22.9) | |
| 12 | 148 (9.5) | 69 (46.6) | 79 (53.4) | 99 (66.9) | 49 (33.1) | |
| 13 | 132 (8.4) | 61 (46.2) | 71 (53.8) | 87 (65.9) | 45 (34.1) | |
| 14 | 134 (8.6) | 73 (54.5) | 61 (45.5) | 103 (76.9) | 31 (23.1) | |
| 15 | 120 (7.7) | 66 (55.0) | 54 (45.0) | 83 (69.2) | 37 (30.8) | |
| 16 | 120 (7.7) | 69 (57.5) | 51 (42.5) | 90 (75.0) | 30 (25.0) | |
| 17 | 80 (5.1) | 42 (52.5) | 38 (47.5) | 67 (83.8) | 13 (16.3) | |
| 18 | 100 (6.4) | 50 (50.0) | 50 (50.0) | 89 (89.0) | 11 (11.0) | |
| 19 | 43 (2.7) | 23 (53.5) | 20 (46.5) | 39 (90.7) | 4 (9.3) | |
| 20 | 7 (0.4) | 2 (28.6) | 5 (71.4) | 7 (100.0) | 0 (0.0) | |
| 21 | 3 (0.2) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | |
| Total | 1,565 (100.0) | 801 (51.2) | 764 (48.8) | 1,160 (74.1) | 405 (25.9) | |
The mean pre- and post-cycloplegic SE was ?1.85 and ?1.19 D respectively. Table 2 shows the distributions of spherical values, cylindrical values and SEs before and after cycloplegic refraction stratified by age (12 years). All these differences before and after cycloplegic refraction were statistically significant (P<0.05). The Kolmogorov-Smirnov tests revealed that none of the parameters were normally distributed. The distribution of SEs before cycloplegic refraction was more skewed towards more myopic values compared with that after cycloplegic refraction. Table 3 compares the crude prevalence estimates of refractive errors including myopia and hyperopia by different definitions based on pre- and post-cycloplegic refraction data. The prevalence estimates are shown in the overall study population and then stratified by age. Using the most common definition of myopia in epidemiologic studies (SE <?0.5 D), the prevalence estimates were 76.7% (95% CI: 74.6–78.8) and 54.1% (95% CI: 51.6–56.6) before and after cycloplegic refraction, respectively. The magnitude of difference was smaller when myopia was defined using a more conservative definition such as SE less than ?0.75 or ?1.0 D. The difference in the prevalence of high myopia before and after cycloplegic refraction was not statistically significant (P=0.15). For hyperopia, when defined as SE of more than 0.5 D, the prevalence was only 2.8% (95% CI: 1.9–3.6) before cycloplegic refraction while it was 15.5% (95% CI: 13.7–17.3) after cycloplegic refraction. The magnitude of difference for clinically significant hyperopia (SE >2.0 D) was smaller (1.4% vs. 0.7%).
| Parameter | Mean (D) | Standard error | Standard deviation (D) | Skewness | Kurtosis | IQR (D) | Kolmogorov-Smirnov test, P |
|---|---|---|---|---|---|---|---|
| Participants aged 12 years or younger | |||||||
| Spherical value | |||||||
| Before cycloplegic refraction | ?0.91 | 0.05 | 1.37 | ?1.11 | 4.45 | 2.00 | <0.001 |
| After cycloplegic refraction | ?0.32 | 0.05 | 1.41 | ?1.25 | 5.28 | 1.25 | <0.001 |
| Cylindrical value | |||||||
| Before cycloplegic refraction | ?0.51 | 0.02 | 0.62 | ?2.62 | 15.39 | 0.50 | <0.001 |
| After cycloplegic refraction | ?0.22 | 0.02 | 0.70 | ?0.99 | 5.58 | 0.75 | <0.001 |
| Spherical equivalent | |||||||
| Before cycloplegic refraction | ?1.17 | 0.05 | 1.45 | ?1.09 | 4.87 | 1.50 | <0.001 |
| After cycloplegic refraction | ?0.43 | 0.05 | 1.56 | ?1.16 | 4.89 | 1.38 | <0.001 |
| Participants aged over 12 years | |||||||
| Spherical value | |||||||
| Before cycloplegic refraction | ?2.32 | 0.07 | 2.02 | ?1.01 | 2.23 | 3.00 | <0.001 |
| After cycloplegic refraction | ?1.78 | 0.07 | 2.03 | ?0.85 | 1.58 | 2.81 | <0.001 |
| Cylindrical value | |||||||
| Before cycloplegic refraction | ?0.58 | 0.02 | 0.60 | ?1.25 | 17.69 | 0.50 | <0.001 |
| After cycloplegic refraction | ?0.52 | 0.03 | 0.68 | ?1.10 | 13.30 | 0.50 | <0.001 |
| Spherical equivalent | |||||||
| Before cycloplegic refraction | ?2.61 | 0.08 | 2.10 | ?1.00 | 2.64 | 2.50 | <0.001 |
| After cycloplegic refraction | ?2.04 | 0.08 | 2.14 | ?0.80 | 1.87 | 2.88 | <0.001 |
D, diopters; IQR, interquartile range.
| Refractive error | Pre-cycloplegic refraction | Post-cycloplegic refraction | P | |||
|---|---|---|---|---|---|---|
| Prevalence (%) | 95% confidence interval | Prevalence (%) | 95% confidence interval | |||
| Myopia | ||||||
| SE < ?0.50 D | 76.7 | 74.6–78.8 | 54.1 | 51.6–56.5 | <0.001 | |
| SE < ?0.75 D | 68.4 | 66.1–70.7 | 49.1 | 46.6–55.6 | <0.001 | |
| SE < ?1.00 D | 61.8 | 59.4–64.2 | 44.5 | 42.0–46.9 | <0.001 | |
| High myopia | ||||||
| SE < ?6.0 D | 3.6 | 2.7–4.5 | 2.7 | 1.9–3.5 | 0.15 | |
| Hyperopia | ||||||
| SE >0.5 D | 2.8 | 1.9–3.6 | 15.5 | 13.7–17.3 | <0.001 | |
| SE >1.0 D | 1.3 | 0.7–1.8 | 4.9 | 3.9–6.0 | <0.001 | |
| SE >2.0 D | 0.7 | 0–1.1 | 1.4 | 0.8–2.0 | 0.04 | |
SE, spherical equivalent; D, diopters.
Based on the post-cycloplegic refraction, the mean spherical equivalent refractive error was ?1.22±1.90 D (median: ?0.50 D, range: ?13.00 to +6.50 D) for right eyes and ?1.30±1.92 D (median: ?0.63 D; range: ?12.75 to +5.75 D) for left eyes, or ?1.38±2.04 D (median ?0.88 D, range: ?13.00 to +6.50 D) for the worse eyes. Refractive errors were not normally distributed (P<0.001).
In univariate analysis, girls as compared with boys were significantly more myopic (?1.48±2.01 vs. ?1.28±2.01 D; P=0.02). Refractive error decreased significantly, i.e., became more myopic, with older age (correlation coefficient r: ?0.43; P<0.001), higher diastolic blood pressure (r: ?0.22; P<0.001) and higher systolic blood pressure (r: ?0.30; P<0.001), lower pulse (r: 0.10; P<0.001), higher body weight (r: ?0.37; P<0.001), taller body height (r: ?0.41; P<0.001) and higher body mass index (r: ?0.25; P<0.001), non-Han ethnicity of father (P=0.001) and mother (P<0.001), higher refractive error of father (r: 0.16; P<0.001) and mother (r: 0.14; P<0.001), and higher number of hours spent indoors (r: ?0.14; P<0.001). Refractive error was not significantly associated with intraocular pressure (P=0.14). The multivariate analysis included all parameters which were significantly associated with refractive error in univariate analysis. Due to collinearity, we first dropped body weight [variance inflation factor (IF): 53.7], body height (IF: 23.6) and systolic blood pressure (IF: 2.2). We then dropped those parameters which were no longer significantly associated with refractive error: pulse (P=0.82), paternal ethnicity (P=0.38), maternal ethnicity (P=0.52), body mass index (P=0.24), In the final model, more myopic refractive errors were significantly associated with older age (P<0.001), female gender (P=0.005), more myopic refractive error of the father (P<0.001) and mother (P<0.001), and less hours spent outdoors after school (P=0.038) (Table 4).
| Parameter | P | Regression coefficient B | 95% confidence interval |
|---|---|---|---|
| Age (years) | <0.001 | ?0.26 | ?0.28, ?0.23 |
| Boys/girls | 0.005 | ?0.26 | ?0.43, ?0.08 |
| Paternal refractive error (diopters) | <0.001 | 0.20 | 0.14, 0.27 |
| Maternal refractive error (diopters) | <0.001 | 0.18 | 0.12, 0.24 |
| Hours spent outdoors after school | 0.038 | 0.18 | 0.01, 0.35 |
The prevalence of myopia defined as refractive errors ≤?0.50, ≤?1.00, and ≤?6.00 D in the worse eye was 60.0%±1.2%, 48.0%±1.3%, and 2.9%±0.4%, respectively. Overall, the prevalence of myopia increased significantly (all P≤0.001) with age (Figures 1,2). In multivariate logistic regression analysis, presence of myopia (defined as myopic refractive error of ≤?1.00 D in the worse eye) was significantly associated with older age (P<0.001), female gender (P=0.001), more myopic refractive error of the father (P=0.002) and mother (P<0.001), and less hours spent outdoors after school (P=0.04).
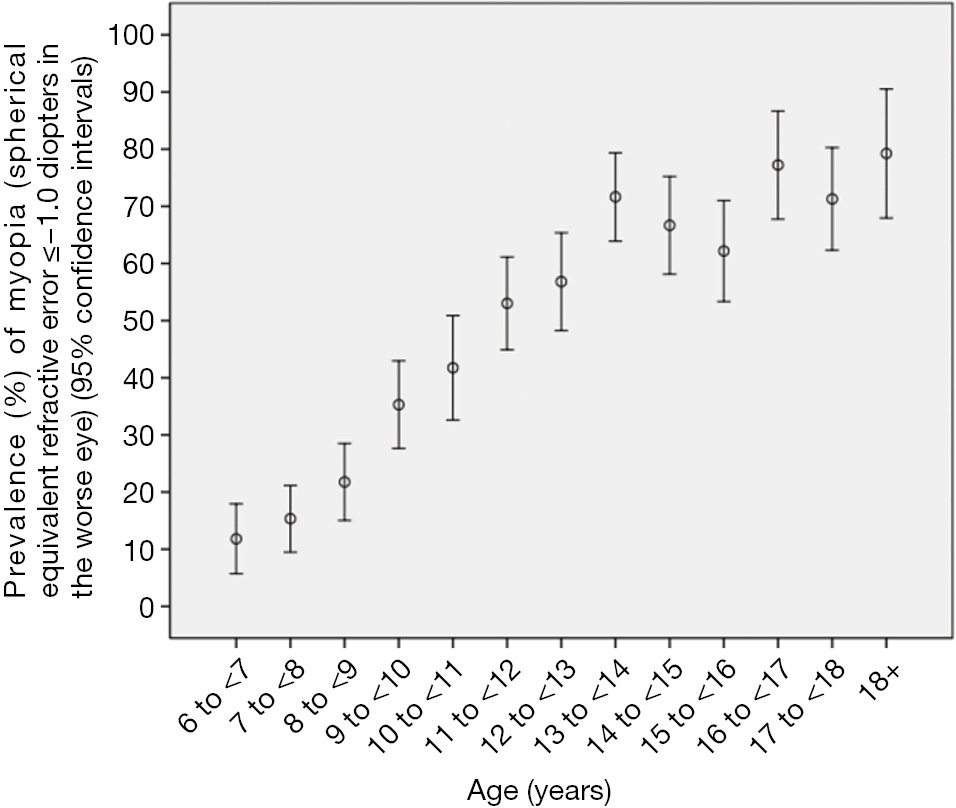
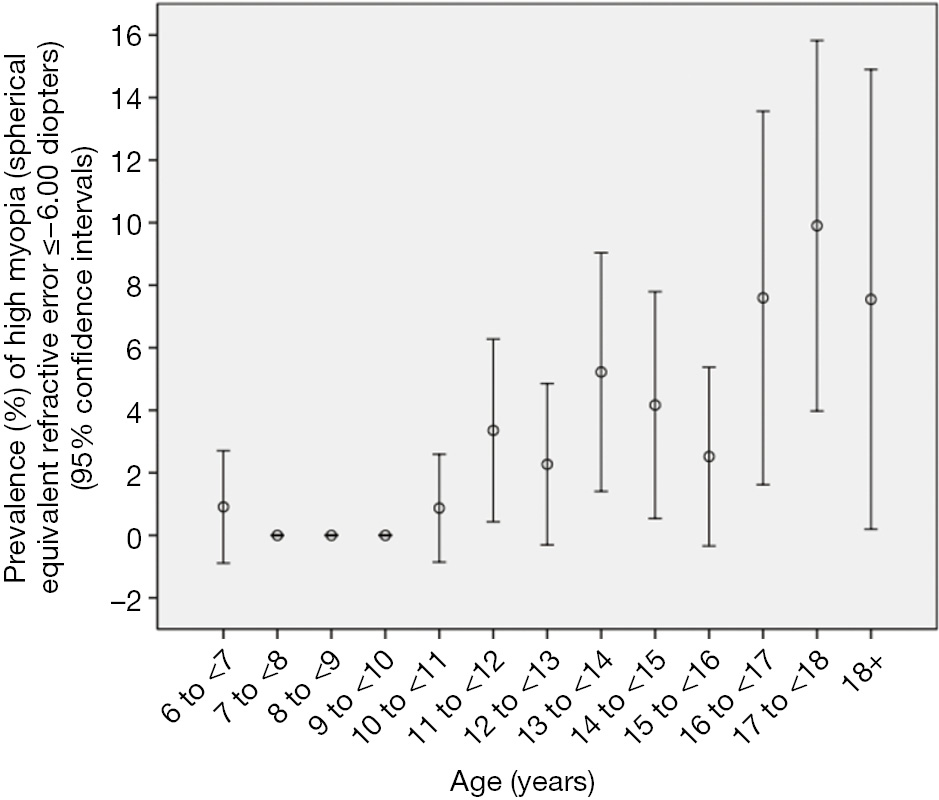
The overall prevalence of high myopia defined as refractive errors ≤?6.00 D was 2.9%±0.4% (95% CI: 2.1, 3.7) (or 45 out of 1,565 study participants). It increased from 3.4%±1.5% in 11-year-old to 9.9%±3.0% in teenagers aged 18+ years (Figure 2). In multivariate logistic regression analysis, presence of high myopia (defined as myopic refractive error of ≤?6.00 D in the worse eye) was significantly associated with older age (P<0.001; OR: 1.37; 95% CI: 1.23, 1.52) and more myopic refractive error of the mother (P<0.001; OR: 0.74; 95% CI: 0.64, 0.85). The mean number of hours daily spent outdoors did not differ significantly between the highly myopic group and the non-highly myopic group (0.93±1.32 vs. 0.88±1.23 hours; P=0.94). Correspondingly, the prevalence of high myopia was not significantly (P=0.66) associated with the number of hours spent outdoors in the multivariate model including age and maternal myopic refractive error. However, the statistical power to detect a significant difference was also limited; it is estimated that a sample size of 4,000 participants would be needed to correctly reject the null hypothesis when it is false.
Out of 1,334 eligible children, 958 (71.8%) children with a baseline mean age of 10.3±2.7 years (range: 6–16 years) participated in the 3-year follow-up study. The incidence of myopia, defined as newly developed refractive error (spherical equivalent) of ≤?0.50, ≤?1.00, and ≤?6.00 D in the worse eye during the 3-year follow-up period, was 52.6%, 45.2%, and 4.5%, respectively. The mean change of refractive error from baseline was ?0.77 and ?0.71 D for the right and left eye respectively. The progression of myopia more than ?1 diopter happened in 51.0% of participants with baseline myopia ≤?0.50 D). The incidence of myopia (≤?1.00 D) significantly increased with older age (P<0.001; B: 1.14), fewer outdoor activity hours (P=0.07, B: 0.66), higher body height (P=0.002, B: 1.02), more myopic paternal refractive error (P<0.02;B: 0.82), but was not significantly associated with gender (P=0.76), ethnicity (P=0.36), baseline intraocular pressure (P=0.86) and choroidal thickness (P=0.19).
Mean IOP was 17.2±3.6 mmHg (median: 16.8 mmHg; range: 5.6 to 31.5 mmHg) in the right eye and 17.2±3.4 mmHg (median: 16.9 mmHg; range: 7.8 to 32.3 mmHg) in the left eye. In multivariate analysis, higher IOP (right eye) was associated with younger age (P<0.001; standardized coefficient beta: 20.13; regression coefficient B: 20.13; 95% CI: 20.18, 20.07), higher diastolic blood pressure (P<0.001; beta: 0.13; B: 0.05; 95% CI: 0.03, 0.07), higher corneal refractive power (P<0.001; beta: 0.11; B: 0.23; 95% CI: 0.12, 0.34), more myopic refractive error (P=0.035; beta: 20.06; B: 20.10; 95% CI: 20.19, 20.001), and Han Chinese ethnicity of the father (P=0.03; beta:0.06; B: 0.42; 95% CI: 0.04, 0.89). If age and diastolic blood pressure were dropped, higher IOP was associated with higher estimated cerebrospinal fluid pressure (CSFP) (P<0.001; beta: 0.09; B: 0.13; 95% CI: 0.06, 0.21) after adjusting for higher corneal refractive power (P<0.001) and Han Chinese ethnicity of the father (P=0.04). Correspondingly, higher IOP of the left eye was associated with younger age (P<0.001; beta: 20.15; B: 20.16; 95% CI: 20.21, 20.10), female gender (P<0.001; beta: 0.09; B: 0.65; 95% CI: 0.30, 1.01), higher corneal refractive power (P<0.001; beta: 0.08; B: 0.19; 95% CI: 0.06, 0.32), more myopic refractive error (P=0.03; beta: 20.06; B: 20.12; 95% CI: 20.22, 20.01), and higher estimated CSFP (P<0.001; beta: 0.11; B: 0.17; 95% CI: 0.09, 0.24).
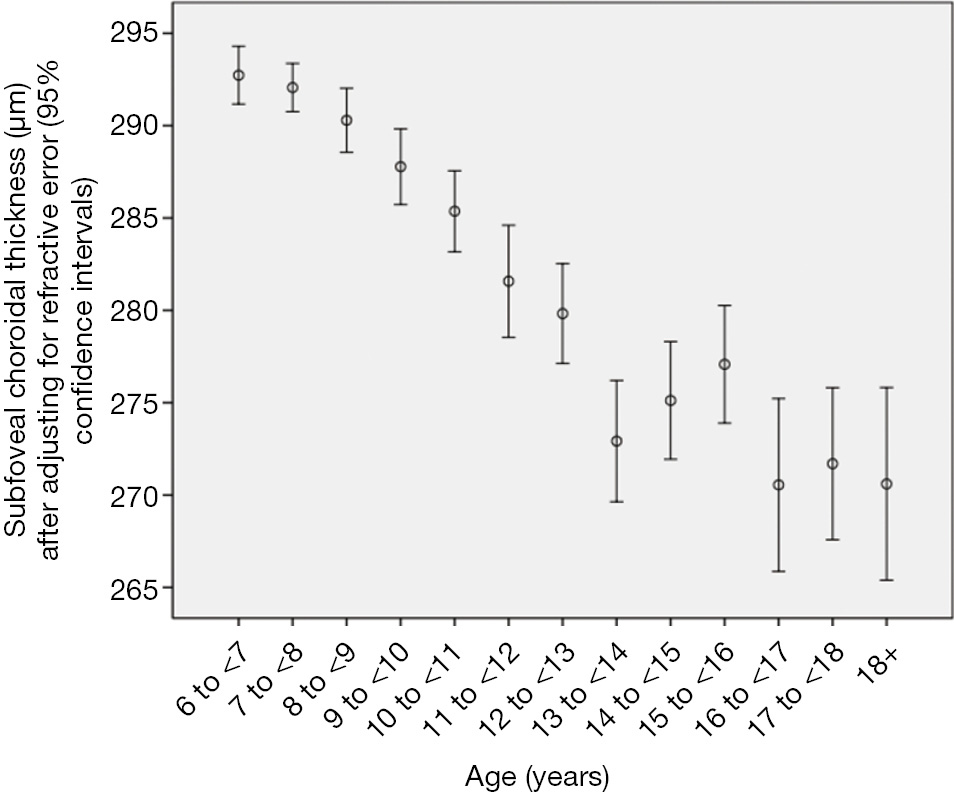
Choroidal thickness (CT) measurements were available for 1,463 (93.5%) students (mean age: 11.8±3.5 years; range: 7–21 years). Mean subfoveal choroidal thickness (SFCT) was 282±49 μm. CT was thickest at 1,000 μm temporal to the fovea (286±49 μm), followed by the subfoveal region (282±49 μm; P<0.001), the region at 2,500 μm temporal to the fovea (278±49 μm), the region at 1,000 μm nasal to the fovea (254±49 μm; P<0.001), and the region at 2,500 μm nasal to the fovea (197±50 μm; P<0.001) (Table 5). In cross-sectional analysis, the mean SFCT increased with age from 288 μm at 7 years of age to 304 μm at 11 years, and then decreased to 258 μm at 18 years. In multivariate analysis, thicker SFCT was associated (regression coefficient r: 0.38) with higher hyperopic refractive error (P<0.001; standardized regression coefficient beta: 0.31; non-standardized regression coefficient B: 7.61; 95% CI: 6.29, 8.93), younger age (P<0.001; beta: ?0.10; B: ?1.39; 95% CI: ?2.14, ?0.64), male gender (P=0.03; beta: ?0.05; B: ?5.33; 95% CI: ?10.1, ?0.53), higher corneal refractive power (P<0.001; beta: 0.12; B: 3.68; 95% CI: 2.12, 5.24), and non-Han Chinese ethnicity (P=0.03; beta: 0.05; B: 6.16; 95% CI: 0.50, 11.8) (Table 6, Figure 3). Ratio of CT (1,000 μm nasal to fovea)/SFCT (0.90±0.06; range: 0.66, 1.23) and ratio of CT (2,500 μm nasal to fovea)/SFCT (0.70±0.13; range:0.28, 1.23) decreased with older age (P=0.01; and P=0.001, respectively), while ratio of CT (1,000 μm temporal to fovea)/SFCT (1.02±0.06; range: 0.56, 1.37) and ratio of CT (2,500 μm temporal to fovea)/SFCT (0.99±0.11; range: 0.54, 1.84) increased with older age (both P<0.001). Time spent outdoors or indoors was not significantly associated with CT-related parameters in multivariate analysis.
| Parameter | Subfoveal choroidal thickness (μm) (right eye) | Choroidal thickness at 1,000 μm nasal to the fovea (right eye) | Choroidal thickness at 2,500 μm nasal to the fovea (right eye) | Choroidal thickness at 1,000 μm temporal to the fovea (right eye) | Choroidal thickness at 2,500 μm temporal to the fovea (right eye) | Subfoveal choroidal thickness (μm) (left eye) | Choroidal thickness at 1,000 μm nasal to the fovea (left eye) | Choroidal thickness at 2,500 μm nasal to the fovea (left eye) | Choroidal thickness at 1,000 μm temporal to the fovea (left eye) | Choroidal thickness at 2,500 μm temporal to the fovea (left eye) |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 1,463 | 1,463 | 1,463 | 1,463 | 1,463 | 1,463 | 1,463 | 1,462 | 1,463 | 1,463 |
| Mean | 282 | 254 | 197 | 286 | 278 | 281 | 256 | 202 | 282 | 268 |
| Median | 284 | 256 | 196 | 287 | 278 | 283 | 257 | 203 | 282 | 269 |
| Standard deviation | 49 | 49 | 50 | 49 | 49 | 51 | 51 | 50 | 50 | 47 |
| Minimal value | 91 | 87 | 60 | 117 | 111 | 105 | 83 | 61 | 26 | 13 |
| Maximum value | 417 | 407 | 388 | 430 | 431 | 473 | 437 | 392 | 466 | 442 |
| Age (years) | n | Mean | Standard deviation | Minimum | Maximum | Prevalence of myopia | Mean spherical equivalent |
|---|---|---|---|---|---|---|---|
| 7 | 108 | 288 | 38 | 196 | 385 | 0.20 | 0.18 |
| 8 | 145 | 290 | 38 | 192 | 386 | 0.23 | 0.05 |
| 9 | 140 | 293 | 44 | 202 | 414 | 0.36 | ?0.20 |
| 10 | 148 | 296 | 45 | 205 | 402 | 0.46 | ?0.51 |
| 11 | 112 | 304 | 44 | 172 | 408 | 0.58 | ?0.81 |
| 12 | 140 | 298 | 55 | 166 | 417 | 0.64 | ?1.30 |
| 13 | 117 | 280 | 54 | 91 | 407 | 0.70 | ?1.52 |
| 14 | 124 | 251 | 51 | 130 | 374 | 0.83 | ?2.31 |
| 15 | 100 | 267 | 43 | 142 | 375 | 0.76 | ?2.17 |
| 16 | 105 | 275 | 54 | 140 | 399 | 0.78 | ?1.90 |
| 17 | 78 | 273 | 47 | 144 | 352 | 0.87 | ?2.58 |
| 18+ | 146 | 258 | 46 | 126 | 362 | 0.78 | ?2.24 |
| Total | 1,463 | 282 | 49 | 91 | 417 | 0.58 | ?1.20 |
Out of 1,565 participants, RNFLT data were available for 1,440 (92%) children (738 boys) with a baseline mean age of 10.3±2.7 years (range: 6–18 years). The mean RNFLT was 101.26 and 101.19 μm for the right and left eye respectively (P=0.83). The RNFLT in the right eye was thickest at temporal inferior (157.32 μm), followed by temporal superior (143.83 μm), nasal inferior (109.74 μm), nasal superior (106.91 μm), temporal (85.18 μm) and nasal (61.67 μm). The distribution pattern of the left eye was the same as the right eye. It was significantly thicker in girls than in boys (101.78 vs. 100.8, P=0.045). In a multivariate analysis, the RNFLT in the right eye significantly increased with refractive diopters (P<0.001, B: 1.595), lower intraocular pressure (P=0.003, B: ?0.021) and female gender (P=0.002, B: 1.444), but not significantly associated with age (P=0.98), body height (P=0.88), systolic blood pressure (P=0.32) and subfoveal choroidal thickness (P=0.09).
In this school-based study, we found that lack of cycloplegia would lead to overestimation of the prevalence of myopia particularly low to moderate myopia, and underestimation of the prevalence of hyperopia. Based on the post-cycloplegic refraction, the study confirms previous studies in that the prevalence and incidence of myopia in general, and that of high myopia in particular, is relatively high in the young generation in China. Prevalence of myopia in the young generation, with figures similar to or even higher than in our study, has been reported for the far more developed urban regions at the Pacific rim of Eastern China (4,5,8,13-18). These studies agree with findings from other countries such as Singapore and Taiwan of high prevalence of myopia in the young generation (19-21). These figures on the prevalence of myopia in the young generation contrast with data on the prevalence of myopia in the elderly generation which was examined in population-based studies such as the Beijing Eye Study. In the latter with a study population aged 40+ years, the prevalence of myopia defined as a myopic refractive error of <?1.0 D was 16.9% (95% CI: 15.8, 18.0), while the 17-year old teenagers in our study had a myopia prevalence of 71.3% (95% CI: 62.3, 80.3) (13). In a similar manner, the prevalence of high myopia (refractive error <?6 D) was 2.6% (95% CI: 2.2, 3.1) in the elderly population of the Beijing Eye Study and it was 9.9% (95% CI: 4.0, 15.8) in the 17-year-olds in our study. Although it is difficult to draw conclusions from comparisons of myopia prevalence figures for study populations, which differ in location, age, examination methods, ethnicity and other parameters, accumulating data suggest that the young generation in China, both at the Pacific rim as well as in Western China, has experienced a marked myopic shift in their refractive errors. While such large increases in the prevalence of myopia have not been observed in Western countries, studies by Vitale et al. and others have reported modest increases in the prevalence of myopia (22-25).
Taking into account that the prevalence of myopia increases with age, the prevalence of myopia in general and of high myopia in particular will further increase in the school children of our study as they get older (4-8,13-18). This has important clinical and public health implications since high myopia is associated with vision threatening disorders such as myopic maculopathy and myopic chronic open-angle glaucoma (3,26,27). Myopic maculopathy is already one of the most important causes for visual field defects and visual impairment in East Asia (20,28,29).
The results of our study can be compared with the findings obtained in previous investigations which were performed in different regions of China at different times. The data reveal an increase in the prevalence of myopia with a higher degree of urbanization and a more recent date of examination. In 1998, the refractive error study in children was conducted in the rural Shunyi district northeast of Beijing on randomly selected 5,884 children aged 5–15 years (4). With an overall prevalence of myopia of 16.7%, myopia was mostly absent in the 5-year-old children, and its prevalence increased to 36.7% in boys and to 55.0% in girls by the age of 15 years. Examining 4,364 children aged 5–15 years in Guangzhou in the year 2004, He and coworkers found a prevalence of myopia of 3.3% in children aged 5 years, and of 73.1% in the children aged 15 years (5). In a study from Hong Kong on 7,560 children aged 5–16 years in 2004, myopia defined as refractive error ≤?0.50 D was found in 36.7%±2.9% of the children (6). In 2005, a similar study was carried out in the southern rural county of Yangxi on 2,454 children with an age of 13 to 17 years (14). Prevalence of myopia increased from 36.8% in the 13-year-old children to 53.9% in the 17-year-old teenagers. In 2010, Pi and colleagues published the results of a population-based refractive error study in the metropolis of Chongqing in West China (15). Prevalence of myopia increased from 0.42% in 6-year old children to 27.1% in 15-year-old teenagers, which is much lower than prevalence reported in other studies. The authors deduced the low prevalence might be related with a lower education intensity and a different living condition in Chongqin. The Beijing Childhood Eye Study carried out in 2008 examined 15,066 school students aged from 7–18 and revealed a prevalence of myopia of overall 64.9%±0.4% (8). Our finding of a high prevalence of myopia in the 18-year-old group agrees with a recent study from South Korea, in which a 19-year-old male population from Seoul had a myopia prevalence of 96.5% (30). Our study also agrees with a recent cross-sectional study on 5,083 students from Donghua University in Shanghai (18). Measured with non-cycloplegic autorefraction, in this educationally selected population, the mean refractive error was ?4.1 D and 95.5% of the students were myopic (<?0.50 D), 19.5% were highly myopic (<?6.0 D), and only 3.3% of the individuals were emmetropic (?0.5 to +0.5 D). The tendency towards a higher prevalence of myopia in the younger generation in China has also been demonstrated in a recent investigation by Xiang and colleagues who showed that the prevalence of myopia was significantly higher in Chinese children than in their parents (16). All these studies including investigations assessing differences in refractive error between parents and their children agree on the considerable increase in the prevalence of myopia (31).
As in previous studies, factors associated with myopia in our study were older age, parental myopia, and time spent outdoors (32-34). Interestingly, the prevalence of high myopia (refractive error ≤?6.00 and ≤?8 D) was not significantly associated with the time spent outdoors in our study. Future investigations are needed to address the question of whether or not the development of high myopia is related to lifestyle, given the limited statistical power to address this question in the current study, resulting from the relatively small sample size. It would have clinical and practical importance, since prolonging times spent outdoors by children has been considered to be a protective measure against the development of myopia in general.
Interestingly, prevalence of myopia and myopia refractive error were not significantly associated with the parental ethnicity (Han Chinese versus Mongolian or other minorities) after adjusting for age, gender, parental refractive error and time spent outdoors. This may suggest that the ethnic background, as compared to lifestyle of the children, parental myopia and gender, played a minor role in the development of myopia. This observation fits with the result of a recent multi-center genetic study in which 24 new loci associated with refractive error were identified and in which a tenfold increased risk of myopia for those individuals carrying the highest number of risk alleles was shown, but in which the genetic variants explained only 3.4% of the phenotypic variation in refractive error (35). These findings emphasize the importance of non-genetic factors in the development of myopia.
In the children of our cross-sectional school-based study, mean choroidal thickness was thickest at 1,000 μm temporal to the fovea (286±49 μm), followed by the subfoveal region (282±49 μm; P<0.001), the region at 2,500 μm temporal to the fovea (278±49 μm), the region at 1,000 μm nasal to the fovea (254±49 μm, P<0.001) and the region at 2,500 μm nasal to the fovea (197±50 μm; P<0.001). In cross-sectional analysis, mean subfoveal choroidal thickness increased with age from 288 μm at 7 years of age to 304 μm at 11 years of age, and then decreased to 258 μm at an age of 18 years. Thicker subfoveal choroidal thickness was associated with higher hyperopic refractive error (P<0.001), younger age (P<0.001), higher corneal refractive power (P<0.001), male gender (P=0.03) and non-Han Chinese ethnicity (P=0.03). Mean ratio of choroidal thickness at locations nasal to the fovea to subfoveal choroidal thickness decreased with older age, while the mean ratio of choroidal thickness at locations temporal to the fovea to subfoveal choroidal thickness increased with older age. Time spent outdoors or time spent indoors was not significantly associated with any choroidal thickness parameter in multivariate analysis.
The mean thickness of the subfoveal choroid in our study population differed from the values reported in some previous studies. In the Copenhagen Child Cohort 2000 Eye Study, mean subfoveal choroidal thickness was 369±81 μm in girls and 348±72 μm in boys, which was higher than the mean value of 282 μm found in our study (36). Potential reasons for the discrepancy may have been differences in age, refractive errors and ethnic background of the study participants. The Copenhagen Study included children aged 11–12 years and with a mean refractive error of +0.1 diopter, while the age of our study population varied from 7 to 21 years and the mean refractive error was ?1.2 diopters. Using Swept source OCT, Nagasawa and colleagues examined 100 healthy Japanese children aged 3–15 years old and reported on a mean choroidal thickness of 260±57 μm (37). The measurement of subfoveal choroidal thickness reported from our study was almost identical to the value found in the Shandong Children Eye Study on 972 children with a mean age of 11.3±3.3 years (range: 6–18 years), a mean axial length of 24.1±1.6 mm (range: 16.6–28.8 mm) and a mean subfoveal choroidal thickness of 283±67 μm (range: 113–507 μm) (38).
As in our study, the Shandong Children Eye Study revealed that the choroidal thickness was thicker (P<0.001) at 500 μm temporal to the foveola (290±67 μm) than in the subfoveal region (283±67 μm) and that it was thinnest (P<0.001) at 500 μm nasal of the foveola (268±67 μm) (38). In the investigation performed by Read and colleagues, 4- to 6-year-old children showed the thickest choroid (322±60 μm) 1.5 mm superior to the foveal center. For the 7- to 9-year-old the mean thickest choroid (344±63 μm) was located in a superior-temporal location 0.8 mm from the foveal center. The thickest choroid of the 10- to 12-year-old (350±58 μm) was located along 0.9 mm temporal to the foveal center (39). Sanchez-Cano and associates reported for young adults, that choroidal thickness was thickest in the region 1.5 mm superior to the foveola, followed by the temporal region and the subfoveal region (40). In another study by Read on children, choroidal thickness was significantly the thickest (346 μm) in the superior region and superior-temporal (341 μm) location at a distance of 1 to 3 mm from the foveal center, and it was thinnest in the nasal region and inferior-nasal (306 μm) area (41). These findings were different from the observations made in adults, in whom the choroid was usually thickest in the subfoveal region, followed by the temporal region and superior region, and in whom choroidal thickness was thinnest in the nasal perifoveal region (42-44). If the regional distribution of choroidal thickness is compared between children and adults, one may infer, as discussed recently, that the fovea of the retina in spatial relationship to the choroid may move into the temporal direction or that choroidal thickness locally adapts to the eventual location of the fovea in adults (38). The increase in the ratio of temporal choroidal thickness to subfoveal choroidal thickness with older age up to an age of at least 18 years as shown in our study population may suggest that the re-arrangement of the choroid in terms of moving the location of the thickest choroidal thickness to the subfoveal region may occur after the age of 18 years. The ratio of nasal choroidal thickness to subfoveal choroidal thickness decreased in our young study population. This may be of interest for the discussion on the development of parapapillary alpha, beta and gamma zones, for which a thinning of the choroid has been described (45,46).
The findings of our study agree with the observations made in previous investigations that choroidal thickness decreased with more myopic refractive error or with a longer axial length as surrogate for myopia, with female gender and with older age (38,39,42,43,47-52). The potential difference between adults and children may be that in adults, choroidal thickness decreased more or less linearly with older age, while in the children of our study population choroidal thickness increased up to an age of 11 years and then started to decrease (Table 2). These results confirm the findings obtained in previous smaller studies. Read and associates reported that the choroidal thickness increased with older age in a group of 194 children with an age of 4–12 years and in another group of 80 children aged 10–15 years (39,41). Bidaut-Garnier et al. examined 174 children with an age of 3.5 to 15 years and also found an increase in choroidal thickness with older age (53). In a longitudinal study on 101 children aged 10 to 15 years observed over an 18-month period, Read and colleagues found a significant (P<0.001) mean increase of 13±22 μm in subfoveal choroidal thickness in hyperopic eyes and in myopic eyes, in addition to an association between thinner choroidal thickness and axial elongation (50). In contrast, Nagasawa and colleagues reported that choroidal thickness decreased with age in their group of 100 children with an age of 3 to 15 years (37). Chhablani and colleagues investigated 136 children with an age of 5–18 years and reported that the choroidal thickness decreased with age (54). Lee and coworkers reported subfoveal choroid is prone to thinning with increasing age in a group of 40 children with an age of 4–17 years (55). In our study with a larger sample size, a larger age range and in particular, with a population-based recruitment of the study participants, the mean subfoveal choroidal thickness increased with older age from 288 μm at the age of 7 years to 304 μm at the age of 11 years, and then started to decrease with further ageing to 258 μm at an age of 18 years. These age-related changes in choroidal thickness in association with age-related changes in choroidal thickness may potentially play a role in the as yet unclear processes of emmetropization and myopization (56,57). Since intraocular pressure may also influence choroidal thickness, and since intraocular pressure also changes with older age in children, future studies may address the inter-relationship between these parameters of axial (optical) length, age, refractive error, intraocular pressure and subfoveal macular choroidal thickness.
As in adults, choroidal thickness in the children of our study population as well as in the populations of other children studies decreased with more myopic refractive error or with longer axial length. The Copenhagen Child Cohort 2000 Eye Study reported that a thinner choroidal thickness was associated with more myopic refractive error or longer axial length (36). Measuring choroidal thickness and axial length in 160 children, Zengin and associates reported that choroidal thickness was negatively associated with axial length (58). Similar findings were reported by Herrera et al. and by Mapelli and coworkers (51,52). In our children study population, subfoveal choroidal thickness decreased by 9.5 μm (95% CI: 7.8, 10.3) for each diopter increase in myopic refractive error in univariate analysis, and by 7.6 μm (95% CI: 6.3, 8.9) for each diopter increase in myopic refractive error in multivariate analysis (Table 4). In the Beijing Eye Study on adult individuals, subfoveal choroidal thickness decreased by 15.7 μm (95% CI: 13.9, 17.5) for every increase in myopic refractive error of 1 diopter beyond a refractive error of ?1 diopter (47).
The associations between male gender and thicker choroidal thickness as found in our study have also been reported for adults and in children. In the Beijing Eye Study and the Singapore Malay Eye Study, subfoveal choroidal thickness was thicker in men than in women (43,46). In the Shandong Children eye Study, thicker choroidal thickness was associated with male gender, while in the study by Bidaut-Garnier and colleagues on a smaller group of children, choroidal thickness was independent of gender (38,53).
In our population-based study on school children in an oasis in the Gobi Desert, higher IOP was significantly associated with younger age, higher diastolic blood pressure, steeper cornea and more myopic refractive error. If diastolic blood pressure were dropped from the analysis in the otherwise unchanged statistical model, higher IOP was significantly (P<0.001) associated with higher estimated CSFP. In the multivariate model, IOP was not significantly associated with BMI.
The association between higher IOP and higher blood pressure as found in our children study was in agreement with previous population based studies on adults, such as the Rotterdam Study, the Singaporean Tanjong Pagar Study, the Blue Mountains Eye Study, the Beaver Dam Eye Study, and the Los Angeles Latino Eye Study (59-64). In univariate analysis, IOP increased by 0.4 mmHg for each increase in diastolic blood pressure by 10 mmHg (Table 2). In the multivariate model, IOP increased by 0.5 mmHg for ach increase in diastolic blood pressure by 10 mmHg (Table 4). Our data thus confirms the results of the preceding studies and extends their findings onto children. It shows that independently of age, IOP and blood pressure are connected to each other.
The association between IOP and steeper cornea (i.e., higher corneal refractive power) again agrees with preceding studies on adults in which similar correlation have been reported (65,66). The higher the corneal refractive power was, i.e., the steeper the cornea was, the higher were the intraocular pressure readings. The finding of our study may be due to geometrical reasons, since a flat structure as compared with a steep structure needs less external pressure to be further flattened up to a standardized applanation area. The clinical importance of the finding is that IOP measurements should be corrected for central corneal thickness and corneal curvature, in after corneal refractive surgery for the correction of myopia.
IOP decreased with older age in our study population (Tables 2-4). These results agree with findings of some studies, and are contradictory to results other investigations. An increase in IOP with older age for 405 children up to an age of 12 years was reported by Sihota and colleagues (67). An increase in IOP with older age for children aged less than 10 years was also reported by Duckman and colleagues (68). In a similar manner, studies reported on different association between higher age and IOP in adults, with increased IOP in Westerners and decreasing IOP with older age in Japanese (69,70).
The limitations of the current study have to be mentioned. First, although the Gobi Desert Children Eye Study has a reasonable response rate of 81.9% at baseline study and 71.8% for 3-year follow-up study, the non-participants might induce a selection bias. Second, the population of the oasis city of Ejina in West China is not representative of China as a whole. Living and other conditions in our study region, however, are similar to those in other regions of the Far West of China, so the results of our investigation may have predictive value for other Western Chinese provinces. Third, we did not measure axial length which would have complemented our data on refractive status measured under cycloplegic conditions. Fourth, the relatively small sample size with a small number of high myopia participants limited the statistical power to address the question whether or not the development of high myopia is related to lifestyle. Strengths of our study were that we included almost all children of the region in contrast to previous school-based studies, in which usually schools were randomly selected and their children were asked to participate in the study.
In conclusion, even in Western China, the prevalence and incidence of myopia in school children is relatively high. As in Eastern China, low and medium myopia was associated with less time spent outdoors. High myopia was not significantly associated with outdoors time. Compared with the myopia prevalence in elderly Chinese populations, the relatively high myopia prevalence in school children overall in China predicts a marked increase in vision-threatening high myopia in the elderly populations in China in the future. The normative data of choroidal thickness, retinal nerve fiber layer thickness and intraocular pressure and their associations reported here provide useful reference for associated clinical practice and future studies on these topics.