Abstract: Diabetic retinopathy (DR) is a leading cause of visual loss worldwide. Disease severity is graded from mild non-proliferative DR to proliferative DR. Optical coherence tomography angiography (OCTA) has become widely accepted as a useful noninvasive technique that provides detailed imaging of the ocular vessels. It is also becoming an increasingly essential tool for both qualitative and quantitative assessment of DR, especially with the advent of wider imaging capabilities. Various angiographic features of DR, such as microaneurysms, intraretinal microvascular abnormalities, neovascularization, and nonperfusion have been comprehensively studied and described using OCTA. Different quantitative OCTA metrics have been introduced, such as vessel density, foveal avascular zone (FAZ) area, and area of nonperfusion. Current research has been focusing on the application of quantitative OCTA for the diagnosis of DR and treatment monitoring. The primary purpose of this article is to review the use of OCTA, including its challenges, in the diagnosis and management of DR.
The worldwide prevalence of diabetes has progressively increased in recent decades and is predicted to grow to 430 million by 2030 (1). Diabetic retinopathy (DR) is a retinal vascular disorder characterized by the development of microaneurysms, retinal hemorrhage, exudate, venous changes and capillary non-perfusion in the non-proliferative stage. The development of neovascularization is the hallmark of progression to the proliferative stage. Grading of disease severity is based on the ETDRS extension of the modified Airlie House Classification (2). The International Clinical DR Disease Severity Scale was proposed with its five-stage classification system: no apparent DR (NDR), mild non-proliferative DR (NPDR), moderate NPDR, severe NPDR, and proliferative DR (PDR) (3). Sight-threatening complications occur as a result of the development of proliferative disease or with macular involvement, such as in diabetic macular edema (DME) and diabetic macular ischemia (DMI) (4).
Fluorescein angiography (FA) has long been the gold standard for the staging of DR. However, it is time-consuming, invasive, and involves the injection of dye, rarely with severe complications. The recent development of optical coherence tomography angiography (OCTA) has allowed for noninvasive three-dimensional imaging of retinal vasculature (5). OCTA is based on a motion contrast technique, which assumes that in a static eye, the only moving aspect is vascular flow. Successive OCT B-scans are taken at the same location and differences between these scans are analyzed and used to generate the OCTA image (6).
Microaneurysms appear in the earliest stages of DR and contribute to disease progression. OCTA has demonstrated an ability to visualize the structure of microaneurysms, although the sensitivity of detection is low as compared to FA (Figure 1) (7). For the MAs that OCTA is able to detect, OCTA can localize precisely the level of the microaneurysm (8). However, a fewer number of microaneurysms are visible on OCTA as compared to FA. This phenomenon may possibly be due to slower flow speeds in microaneurysms that are beyond the OCTA detection threshold (9). Another hypothesis suggests that focal staining of vessel walls allows for superior identification in FA (10). However, OCTA has been demonstrated as more effective in detecting microaneurysms compared to color fundus photography (11). The automated detection of microaneurysms is somewhat difficult, due to their small size and continuity with blood vessels (12). However, their automated detection has been demonstrated in color fundus photography and OCTA, for which algorithms continue to be developed (13,14).
OCTA has allowed us to visualize the morphology of IRMA using en face images. IRMAs are seen on OCTA as dilated or looping vessels near areas of capillary loss and their detection on OCTA has been demonstrated as less challenging than on color fundus photography, where they may not be as well-defined. OCTA has demonstrated that the caliber of these loops was noticeably greater than that of surrounding normal capillaries, thereby allowing easier distinction from normal vasculature (11). OCT has further described features associated with IRMA, such as the presence of intraretinal hyperreflective dots and outpouching of the internal limiting membrane (15,16).
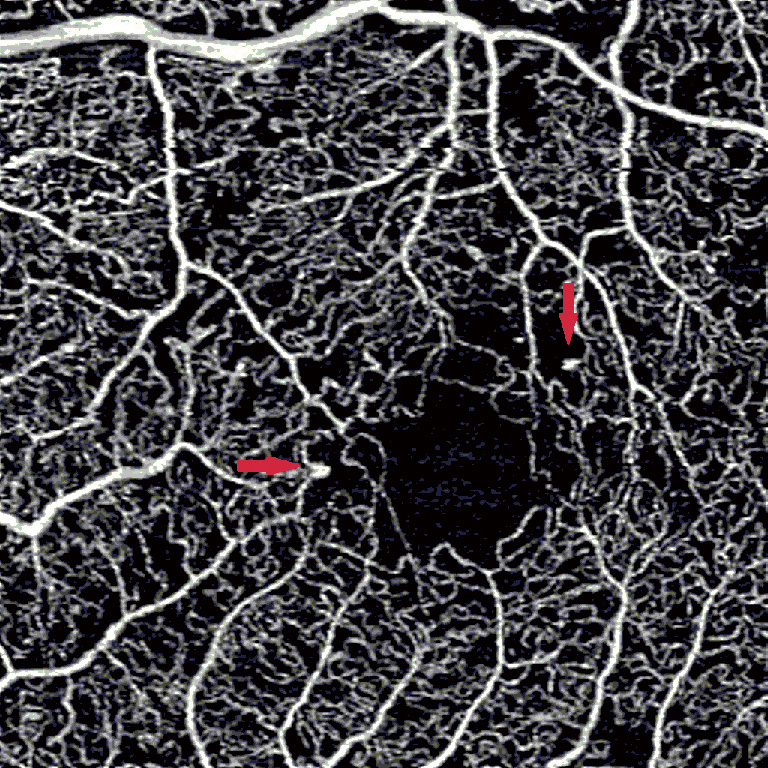
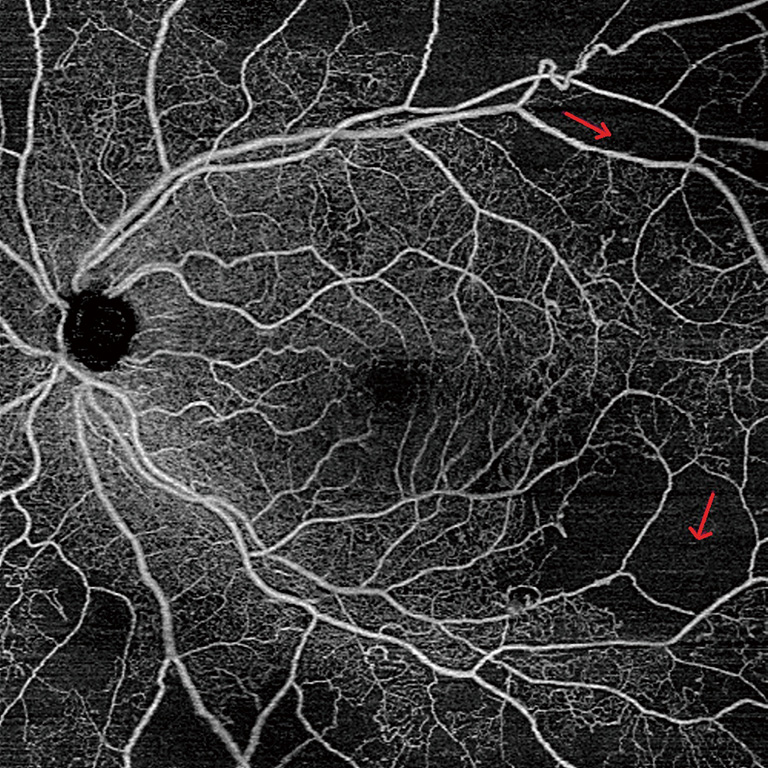
Compared to IRMA, neovascularization is associated on OCT with a disruption of the ILM (15). OCTA allows for the detection of retinal neovascularization by observing flow signal above the internal limiting membrane (16). OCTA has also enabled the identification of subtle NV, indistinguishable in FA from a microaneurysm (17). However, the focus depth range of OCTA limits the visualization of highly elevated NV that extends substantially beyond the margin of the ILM. Due to the limited focusing ability of OCTA, such NV may present as shadow rather than flow (17). However, the manual identification of neovascularization may be more time consuming on OCTA as compared to visualization of a leaking NV on FA (18). Moreover, leakage on FA may obscure the margins of NV. Since there is no leakage on OCTA, it is possible to precisely measure the size of NV and then follow it up over time, watching it regress after anti-vascular growth factor or panretinal photocoagulation. Overall, OCTA provides additional information regarding the localization and morphology of NV, which may be useful in determining disease severity and treatment (Figure 2) (19).
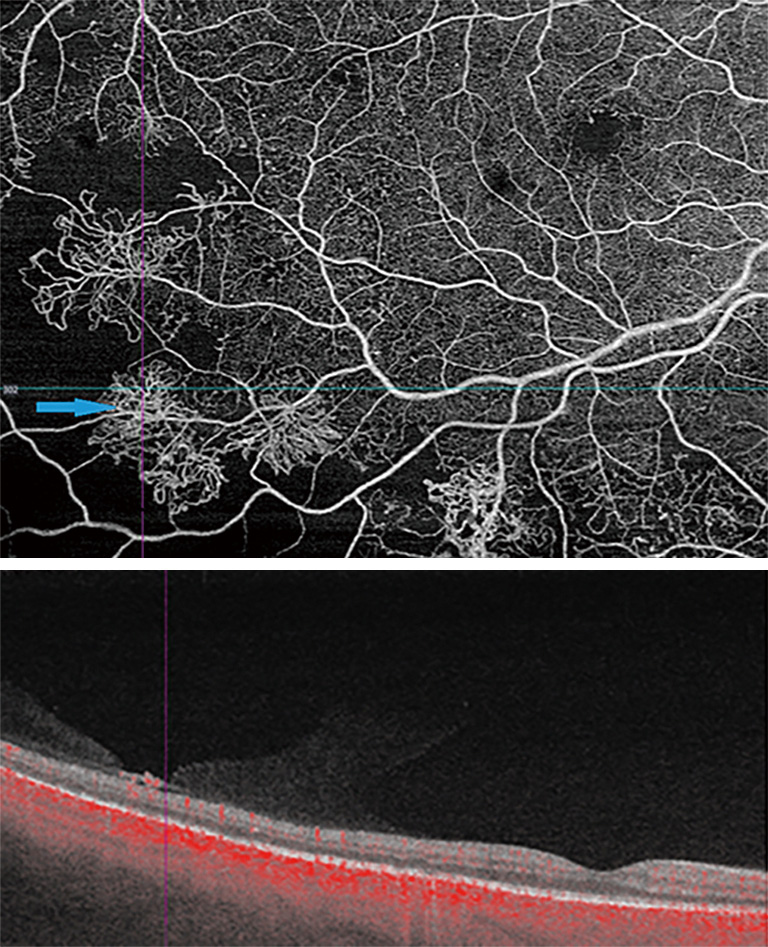
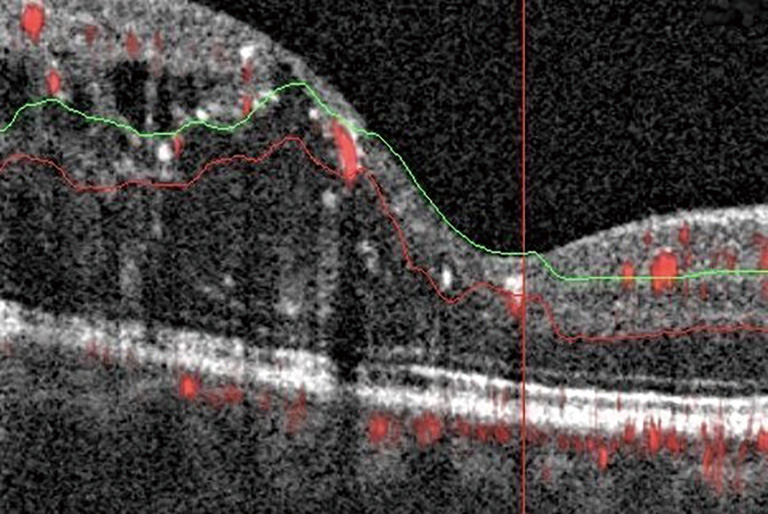
DME develops due to a combination of both VEGF-mediated and inflammatory factors, resulting in alterations in the blood-retinal barrier. The subsequent presence of fluid on OCT may interfere with imaging and segmentation capabilities. This occurs because of difficult automated identification of anatomical landmarks needed for correct segmentation (Figure 3) (20). Incorrect segmentation may affect OCTA images. DME has also been correlated with decreased OCTA signal intensity (9) as the cystoid spaces in DME often attenuate the reflected signal from deeper layers (21). Areas of DME may appear as flow voids on OCTA, areas that lack flow signal. It has been reported that these flow voids on OCTA were frequently larger than the cystoid spaces themselves, giving an inaccurate representation of actual morphology (Figure 4) (22). Studies have also determined the density of microaneurysms in superficial and deep retinal layers. Microaneurysms were found to be more frequent in the deep layer as compared to the superficial layer in eyes with DR. Their density in the deep layer further increases in eyes with DME. Additionally, a correlation between macular volume and the density of microaneurysms in the deep plexus has been demonstrated (23).
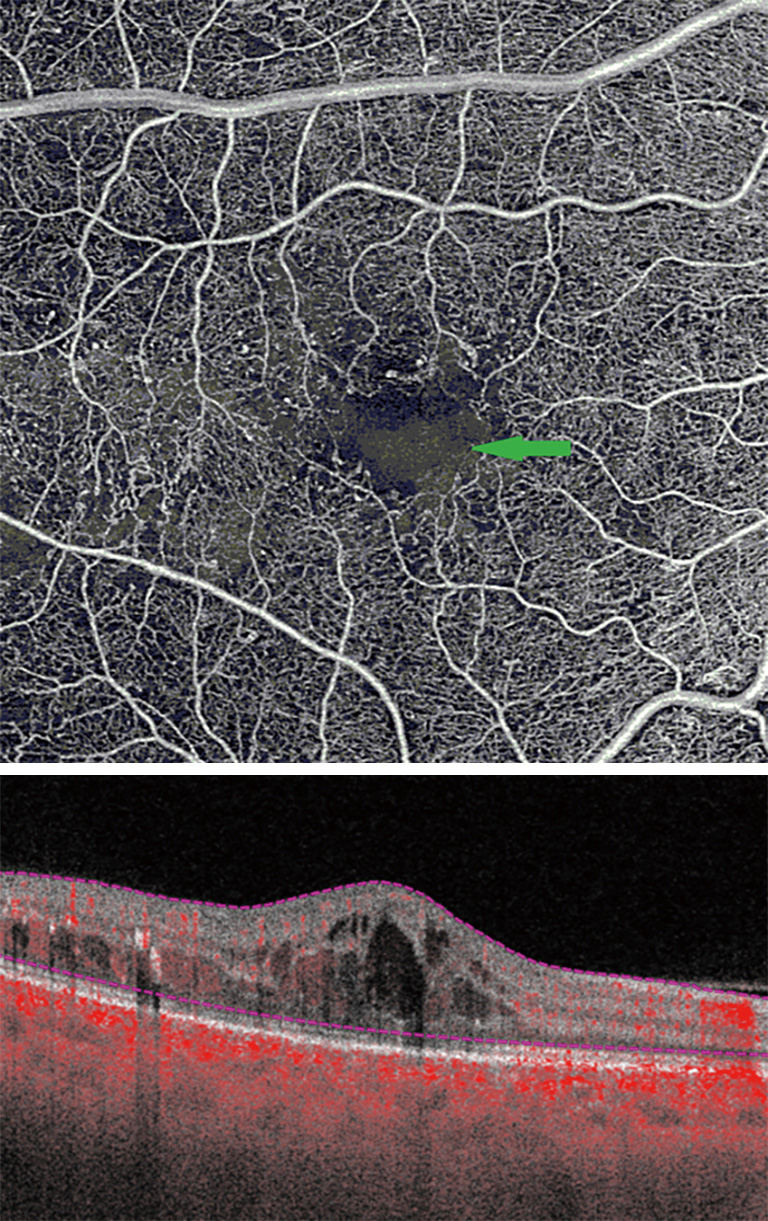
FA has previously demonstrated enlargement of the FAZ in patients with DR as compared to normal, as well as progressive enlargement with progression of DR severity. OCTA has further confirmed these findings (24). Various quantitative FAZ metrics on OCTA have been used to demonstrate these trends, such as FAZ area, axis ratio, acircularity index, area, and perimeter (Figure 5) (25). Acircularity index has been de?ned as the ratio of the perimeter of the FAZ to the perimeter of a circle with equal area (26). Axis ratio was defined as the ratio between the major and minor axis of the FAZ. Both acircularity index and axis ratio were found to be significantly greater in eyes with DR compared to normal and diabetic eyes with no DR. Furthermore, there was also a significant difference in acircularity index between NPDR and PDR patients, while no differences were observed between normal eyes and diabetic eyes with no DR (27).
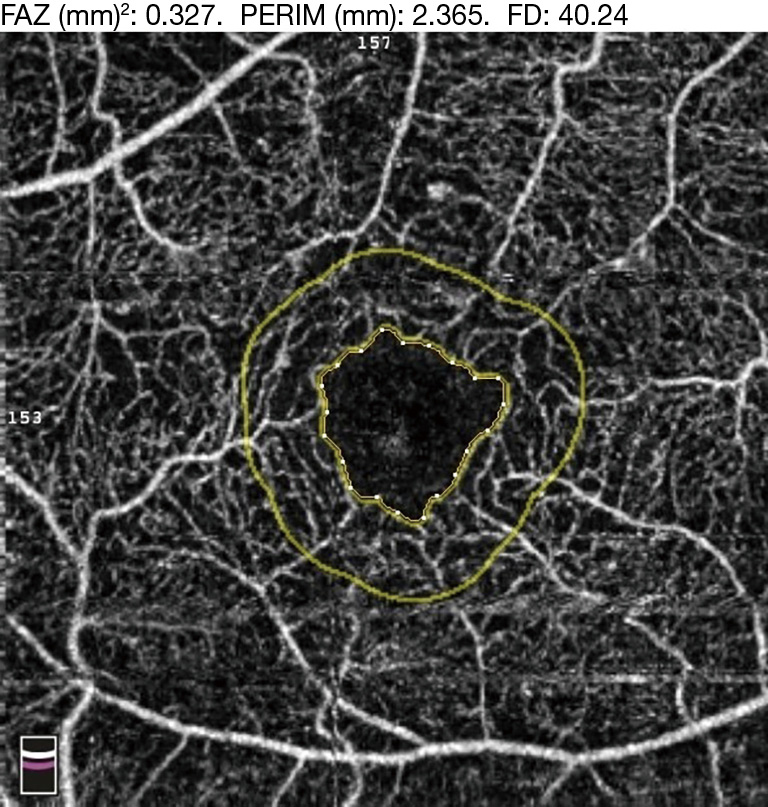
FA images have used FAZ contour to grade for ischemia in DR (28). When the same grading system was applied to OCTA, good agreement was demonstrated between both modalities, indicating that OCTA could be a useful tool for the screening of DMI (29). Similarly, compared to adaptive optics fluorescein angiography (AOFA), OCTA has again demonstrated good agreement in measurements of FAZ metrics (30).
Additionally, an OCTA study was performed analyzing disorganization of inner retinal layers (DRIL) in eyes with resolution of DME. Significantly, larger FAZ area was seen in eyes with DRIL as compared to DME-resolved eyes without DRIL. Areas of non-perfusion on the OCTA also appeared to correspond to areas of DRIL on co-registered structural OCT B-scans (31).
DMI is a common finding in DR. Capillary nonperfusion impairs the nutrition of neuroglial tissues. The resultant hypoxia increases the expression of vascular endothelial growth factor (VEGF). This promotes both angiogenic responses and vascular permeability, thereby causing formation of vascular abnormalities, such as IRMA, NV, and DME (Figure 6).
To quantify non-perfusion, manual tracing has been used. Manual tracing of the non-perfused areas in the superficial and deep capillary plexuses has demonstrated that the average area of retinal non-perfusion in the superficial plexus was greater than that in the deep plexus (8). However, manual tracing is time-consuming, and to overcome this aspect, more automated techniques to quantify nonperfusion have recently been explored.
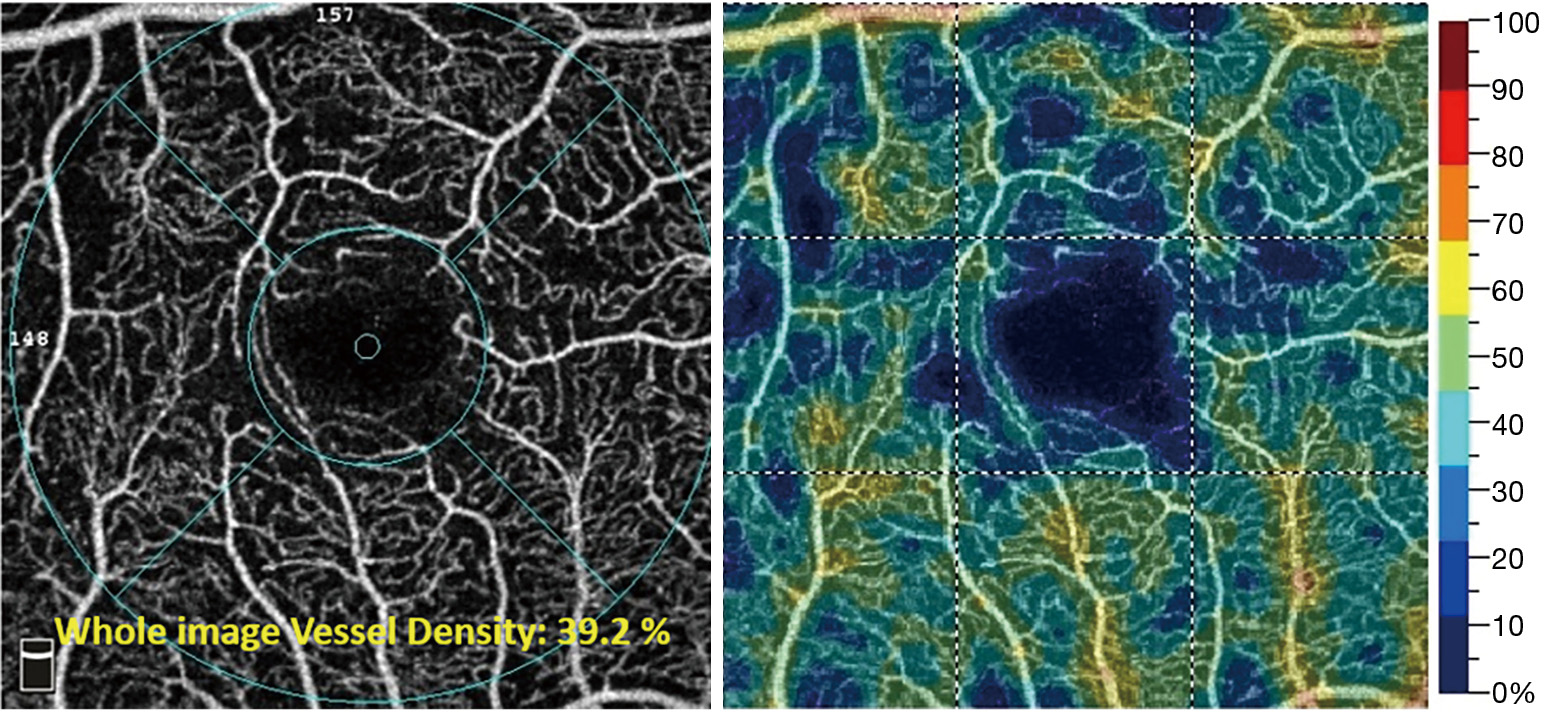
Vessel density (the percentage of area occupied by vessels in an OCTA en face image) and flow index (the average OCTA “decorrelation” flow signal) are quantitative metrics that have also been used to assess for ischemia (Figure 7) (32). However, these threshold-based approaches have significant limitations when applied to images with low signal-to-noise ratios or varying signal strengths (33).
A derivative of vessel density is vessel skeleton density. Skeleton density essentially measures the “length” of vessels in the OCTA images and removes the potential confounding influence of vessel diameter on measurement, making it a more sensitive approach for estimation of retinal non-perfusion (34). With progressing DR severity, vessel skeleton density values have been shown to decrease for both the superficial capillary plexus and deep capillary plexus. Both vessel density and skeleton density demonstrate the same trend of decreasing values with increasing severity of DR (35).
As another quantitative metric, perifoveal intercapillary area has also been used to measure ischemia. Perifoveal capillary area has been measured as areas of no flow contiguous with the FAZ after delineation of the FAZ boundary (36). Similar to the quantitative metrics discussed above, perifoveal intercapillary area has also demonstrated an increase with progression of DR, indicating increasing nonperfusion (36). Schottenhamml et al. further improved this approach by quantifying total intercapillary area using an automated algorithm. Although this approach is more sensitive to small capillary changes, small errors in segmentation may potentially cause large errors in the measured intercapillary area (37).
Fractal dimension is another metric that quantifies the complexity of the vascular network and has been applied in eyes with DR using OCTA. Its measurement in both the superficial and deep capillary plexuses showed a significant difference in diabetics compared to normal. However, the metric did not vary among different severity levels of DR (38).
OCTA depicts binary information with either the presence or absence of flow. To overcome this limitation, variable interscan time analysis (VISTA) was developed to depict relative blood flow speeds. In currently available spectral-domain OCTA devices, the time interval between successive B-scans is ~5 ms. Flow slower than this interval would not be detected via conventional OCTA. Faster flow speeds higher than a certain threshold may cause image saturation. VISTA overcomes this limitation of OCTA. VISTA has been employed on a swept-source OCTA prototype device with faster scanning speeds (~400,000 A-scans per second). Faster scanning speed allows for the acquisition of a greater number of successive B-scans at a certain location without increasing imaging time. The VISTA algorithm is then able to assess the decorrelation signal between consecutive B-scans (~1.5 ms), as well as between alternative B-scans, with an interscan time of ~3 ms. This allows for an improved assessment of slower blood flow speeds, while also maintaining a reasonable threshold for the fastest detectable flow (39). This decorrelation signal is then mapped onto a color display that demonstrates relative retinal blood flow speeds (40). VISTA has been employed to analyze certain vascular features of DR, such as neovascularization and microaneurysms. The application of VISTA has enhanced the visualization of neovascularization by showing more vascular detail. Furthermore, neovascularization has demonstrated low relative blood flow speeds. Capillary loops and microaneurysms were also associated with slower relative blood flow speeds (41).
Histological analysis of eyes with DR has revealed that pathological changes may occur long before the appearance of any clinical manifestations. Thus, conventional grading may detect the disease at a more advanced stage (42). However, OCTA may offer an opportunity to detect this DR while it is still subclinical.
However, while it is easy to view the vascular changes in diabetics without retinopathy, quantifying these changes can be challenging. One study compared various quantitative metrics in OCTA between normal eyes and those of diabetics without DR. Quantitative and qualitative analysis looked at capillary dropout, vessel tortuosity, FAZ area, vessel density, and intercapillary area. This study did not find any significant differences between the two study groups in any of these comparisons (43). Even in a cohort combining diabetic eyes with no DR with eyes with mild NPDR, no significant differences were noted between this cohort and normal controls in either the superficial or deep plexuses. However, a significant decrease in deep plexus vessel density was found between normal eyes and those with mild NPDR. Overall, this study suggested that perhaps OCTA may not yet be a suitable tool to detect subclinical DR (44). In contrast, other studies have demonstrated the opposite. For example, using OCTA, de Carlo et.al have demonstrated an enlargement of the FAZ in diabetics without DR as compared to normals (45). Another study had similar findings in the superficial capillary plexus. This study also found that compared to normal eyes, parafoveal vessel density is decreased in the superficial and deep vascular layers in diabetic eyes with no DR (46). Agemy et al. had similar findings measuring capillary perfusion density in normal eyes and diabetic eyes with no DR (34).
In addition to perfusion density, Durbin et al. also used skeletonized vessel density with similar findings in both superficial and deep retinal layers. Interestingly, vessel density measured in the superficial layer had the greatest area under the curve, suggesting that it might be a more superior parameter for differentiating healthy eyes from eyes with DR (47). Overall, these studies demonstrate that perhaps OCTA may eventually become a useful detection tool for subclinical DR.
In addition to distinguishing normal eyes from those with DR, quantitative OCTA may also be useful in staging DR severity. Vessel density, skeleton density, and fractal dimension have demonstrated a negative correlation with increasing severity of DR (48). Al-Sheikh et al. had similar findings with vessel density and also measured FAZ area with an increasing trend. They additionally noted that parameters from the deep capillary plexus showed greater efficacy in the context of disease stratification (49). Other studies have even utilized artificial intelligence with OCTA imaging. Sandhu et al studied this concept with vascular density, vessel caliber, and FAZ size in both superficial and deep plexuses. Automated diagnosis of disease severity with artificial intelligence achieved an accuracy of 94.3% with area under the curve of 92.4%, suggesting robust measurement value (50).
OCTA may allow us to better understand the effects of laser treatment and anti-VEGF therapy in eyes with DR. Cole et al. demonstrated areas of increased OCTA signal in the choroid in patients that received focal laser, thereby depicting the structural and angiographic changes that occur with laser therapy (51).
The role of intravitreal anti-VEGF injection in the treatment of DME has been well-established (52). FA has demonstrated the efficacy of anti-VEGF in slowing the rate of progression of or even resolving retinal non-perfusion (53). In contrast, OCTA studies comparing FAZ area and vessel density before and after anti-VEGF treatment in eyes with DR have shown that there is no difference in these metrics before and after therapy (54). However, this study examined the effects of a single injection, and thus, one month may not be a sufficient study period to observe perfusion changes. Additional studies have demonstrated no difference in macular vessel density measured along the course of three intravitreal injections. However, treatment was associated with substantial improvement in central retinal thickness (55). These studies seem to suggest that good clinical response may not necessarily correlate with an improvement of microvascular changes seen on OCTA. Certain eyes may be resistant to anti-VEGF treatment. DME eyes that responded poorly to treatment demonstrated a significantly lower flow density, a greater number of microaneurysms, and a larger deep plexus FAZ area (56).
In addition to these studies assessing anti-VEGF treatment, vessel density has also been measured before and after an intravitreal dexamethasone implant for DME. No changes were found in superficial and deep vessel densities. However, choriocapillaris vessel density showed a tendency to increase post-treatment (57), although this may be simply because of better signal penetration.
Substantial variation exists between the various quantitative metrics of OCTA across devices, with each device employing their own proprietary algorithms. This is a major limitation for comparison across devices and between patient follow-ups. Possible sources of this variation include non-standardized segmentations dependent on device-manufacturer algorithms, and inaccurate automated segmentation in the presence of retinal pathology, such as DME. Further contributing to this variability are differences in computational methods of a specific metric between studies.
Additionally, OCTA is prone to artifacts. Superficial retinal vessels cause projection artifacts, in which they falsely appear in deeper retinal layers. Projection artifact removal algorithms have been developed to overcome this limitation. These algorithms often work by subtracting the superficial en face OCTA from the deeper layers. However, this technique may result in loss of flow information in the deeper layer, giving rise to a disconnected image (58). OCTA is also sensitive to motion artifacts. Motion artifacts appear as a white line across the image and may markedly affect imaging quality, and thus quantification as well. To overcome this limitation, recent advances in OCTA have applied motion correction and eye-tracking algorithms (59).
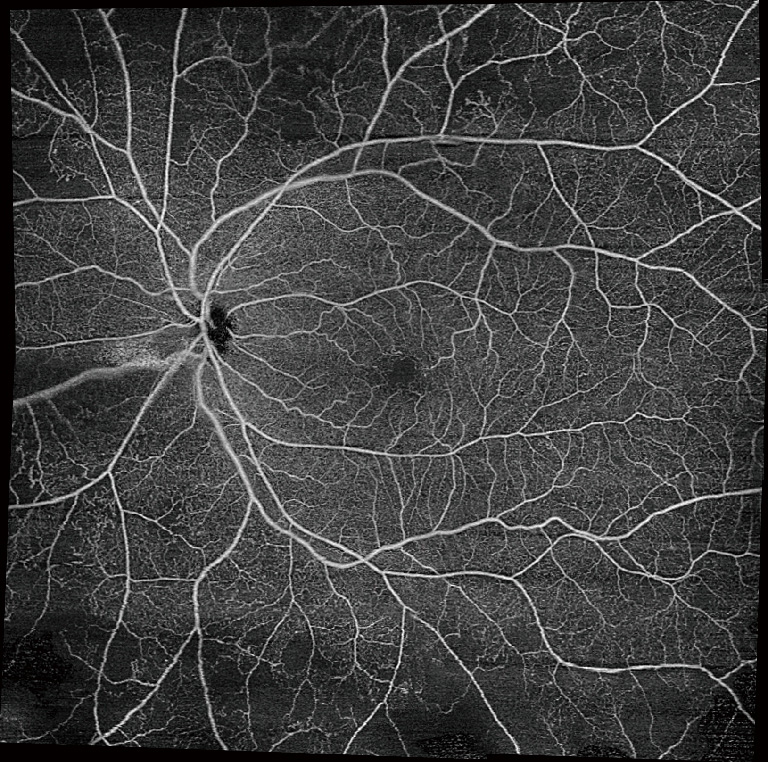
Another limitation of OCTA is a restricted field of view. Commonly used scan patterns range from 3×3 to 12×12 to 15×9. However, these maximum scan sizes are far from comparable to ultra-widefield angiography. Studies have suggested that widefield imaging may reveal more pathological features compared to the classic seven ETDRS fields. To increase the field of view of OCTA, montaging algorithms have been developed, including semi-automated ones in some commercial devices. The 12×12 mm scans can be montaged to generate a wider field of view (Figure 8). Keeping in mind the advantages of OCTA, the possibilities of widefield OCTA seem endless. However, montaging requires a substantially increased scan acquisition time to obtain multiple scans. Additionally, the technique may suffer from inaccuracies due to subtle misalignments (60). As another method to capture a wider field of view, a recent study using SS-OCTA with a subsidiary +20 D lens was able to capture a 55° field of view, even larger than that of FA (61).
Despite its limitations, OCTA demonstrates the ability to provide crucial information about vascular changes in varying stages of DR. This has the possibility to allow for treatment monitoring, screening for DR, and assessment of disease severity. Overall, OCTA has helped increase our understanding of the microvascular changes involved in DR and holds further promise in revolutionizing our assessment of the disease.