The standard treatment for pterygium was surgical excision with bare sclera technique which was associated with a high recurrence rate. Therefore adjunct treatment options are used to reduce this recurrence, such as the use of beta radiation, conjunctiva auto graft, amniotic membrane grafting and mitomycin C (1,2).
Pterygium recurrence is one of the challenging problem facing ophthalmologists due to multiple factors related to vascular component of the pterygium and corneal stem cells activity. The recurrence of pterygium could occur due to fibroblast proliferation and corneal neovascularization as parts of healing process following conjunctiva excision (3,4). Corneal neovascularization secondary to the traumatic and inflammatory process following pterygium surgery is one of the triggering factors for pterygium recurrence. Another hypothesis is that loss of limbal stem cell barrier function allows conjunctiva to grow over cornea. Therefore adjunct treatment aims at inhibiting the fibroblast proliferation or covering the bare sclera with a tissue of similar properties (5).
Vascular endothelial growth factor (VGEF) is an angiogenic factor in the cornea and is considered the trigger factor for corneal neovascularization and hence pterygium recurrence. The use of topical or sub conjunctiva bevacizumab which is an anti (VEGF) is relatively safe and well tolerated in pterygium surgery in prevention of recurrence (6,7). However Miraftabi and Nilforushan [2016] (8) during studying the adverse effects of sub conjunctiva injection of anti-VEGF, they stated that sub conjunctiva injection of bevacizumab may result in delayed conjunctiva healing and necrosis.
The prospective randomized comparative study was performed at the Zagazig University Hospital, Zagazig University, Egypt and included 60 eyes of 60 patients with recurrent pterygium for 1 time. The study was performed according to WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Also the protocol of the study was approved by the ethical committee of Faculty of Medicine Zagazig University. Written consent was obtained from the patients and included an explanation of the study and agreement to have a photo of the eye area only taken. All cases were subjected to:
Postoperatively the cases were divided randomly into two equal groups. Group A which included (30 eyes of 30 patients) was treated with postoperative topical tobramycine and dexamethasone (Tobradex, Alcon Inc., USA) 4 times daily for 2 weeks. Group B (30 eyes of 30 patients) was treated also with postoperative topical tobramycine and dexamethazone in addition to topical bevacizumab (Avastin 100 mg/4 mL, Roche Ltd., Basel) which prepared in polyvinyl alcohol 1.4 (W/V) (Liquifilm Tears, Allergan Ireland) to reach the concentration of 5 mg/mL and used 4 times daily for 2 weeks, according to Kaja et al. [2011] (9), the bottle was kept in refrigerator (4–8 °C) for the stability and sterilization of bevacizumab material. Follow-up of cases weekly up to 6 months was performed recording corneal neovascularization from the limbus using the slit lamp scale in measuring the length of the vessels inside the cornea in millimeters and pterygium recurrence, also patient complaint from any toxic side effects as pain and burning sensation. Statistical analysis of the data was performed using the Z and Chi square tests.
The demographic data on patients in group A revealed that 19 patients (63.3%) were male and that 11 patients (36.7%) were female. Age of the patients ranged from 29 to 72 years with a mean age of 48.6±9.8 years. Group B revealed that 20 patients (66.7%) were male and that 10 patients (33.3%) were female. Age of the patients ranged from 32 to 69 years with a mean age of 50.3±11.6 years.
The postoperative results revealed that after 6 months of follow-up: in group A treated postoperatively by tobramycine and dexamethazone, among the 30 eyes there were 5 eyes (16.7%) showed recurrence which was not significantly different from group B treated by the addition of topical bevacizumab which recorded 3 eyes (10%) P=0.44726 (>0.05).
Regarding to corneal neovascularization, (by measuring the vessels length inside the cornea using the slit lamp scale) in group A, there was corneal neovascularization more than 3 mm from the limbus in 9 eyes (30%) including the recurrent 5 eyes which was significantly different from group B 2 eyes (6.6%) P=0.01928 (<0.05), corneal neovascularization from 2–3 mm in group A 8 eyes (26.7%) which was significantly different from group B in 1 eye (5.5%) P=0.0114 (<0.05), corneal neovascularization from 1–2 mm, group A in 7 eyes (23.3%) which was not significantly different from group B in 8 eyes (26.7%) P=0.76418 (>0.05) and no corneal neovascularization in group A in 6 eyes (30%) which was statistical highly different from the group B in 19 eyes (63.3%) P=0.00068 (<0.05), Total P value concerning the presence and absence of vascularizationbetween the two groups was P=0.000805 (>0.05) which was highly significant (Table 1), (Figures 1,2).
| Parameters | Group A | Group B | P value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Pterygium recurrence | 5 | 16.7 | 3 | 10.0 | 0.44726 (>0.05) | |
| No corneal neovascularization | 6 | 30.0 | 19 | 63.3 | 0.00068 (<0.05)* | |
| Corneal neovascularization 1–2 mm | 7 | 23.3 | 8 | 26.7 | 0.76418 (>0.05)* | |
| Corneal neovascularization 2.1–3 mm | 8 | 26.7 | 1 | 5.5 | 0.01140 (<0.05)* | |
| Corneal neovascularization >3 mm | 9 | 30.0 | 2 | 6.6 | 0.01928 (<0.05)* | |
P<0.05=significant, P>0.05=insignificant. *, total P value is 0.000805. The result is significant at P<0.05 (concerning the presence and absence of vascularization).
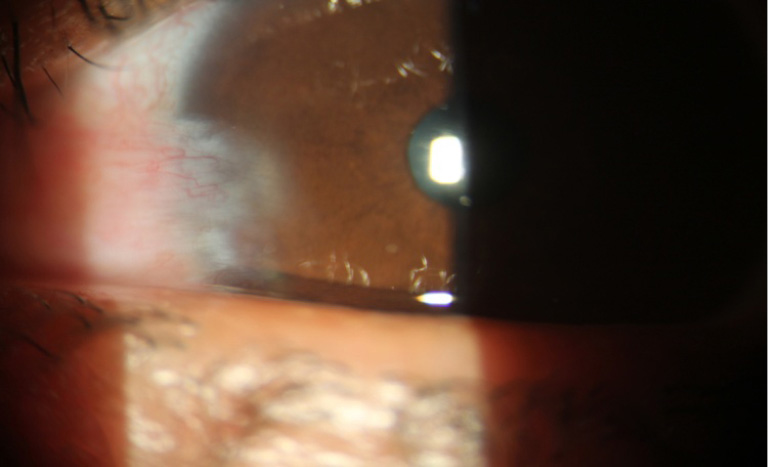
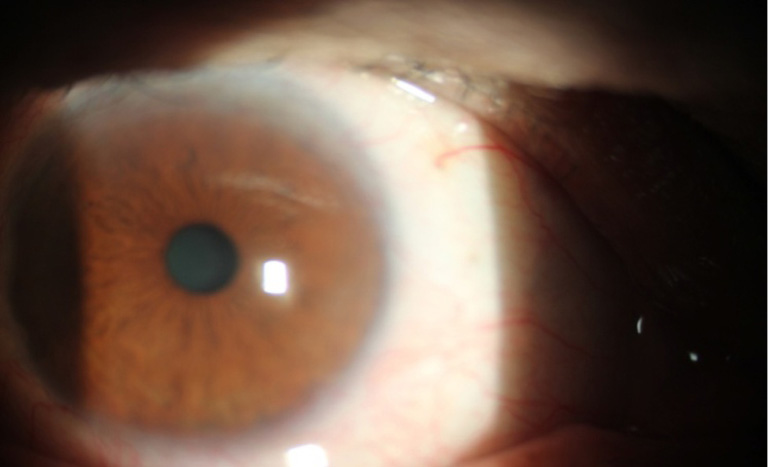
Among the 5 recurrent cases in group A, 4 cases were between 32–49 years, comparing to the 3 cases in group B between 46–65 years.
There was no eyes recorded any toxic side effects as pain, burning sensation, however 2 eyes in group A revealed graft necrosis.
The study was designed to evaluate the efficacy of postoperative short-term therapy for 2 weeks of topical bevacizumab in adjunct to pterygium excision with conjunctiva auto graft in recurrent pterygium surgery, The choice of this duration was based on Kaja et al. [2011] (9) to get the best duration for stability of the diluted bevacizumab, also topical use was preferred to be a non invasive route, also for avoidance of healing disorders and necrosis of the conjunctiva graft according to Miraftabi and Nilforushan [2016] (8). Pterygium excision with conjunctiva auto graft was considered the stander operation for both primary and recurrent pterygium. As the present study did not record a significant difference in recurrence rate between the two groups reporting 16.7% in group A and 10% in group B, it may be explained by the choice of conjunctiva auto graft procedure as it represented a lower rate in recurrence of pterygium specially in recurrent cases as reported by Salagar and Biradar [2013] (10) who reported (6%) recurrence rate among 100 eyes operated by conjunctiva auto graft. Also, the present study reported that the majority of recurrent cases in group A were younger than cases in group B denoting that the age of the patients may play another role in pterygium recurrence.
Concerning to the use of postoperative topical bevacizumab, The present study also agreed with the study of Ozgurhan et al. [2013] (6) who reported (9%) recurrence rate which also was not significant different than the present study as they used the same concentration but for longer duration of one month postoperatively, also nearly with same results of the study of Hu et al. [2014] (7) who carried out their study in 482 eyes and they reported that topical and sub conjunctiva bevacizumab had no statistically significant effect on preventing pterygium recurrence, Even the use of sub conjunctiva injection bevacizumab did also the same results in pterygium recurrence according to the study of Shenasi et al. [2011] (11).
This study demonstrated the high efficacy of topical bevacizumab 5 mg/mL in minimizing corneal neovascularization in agreement with many studies such as the Kim et al. [2013] (12) and Ozdemir et al. [2014] (13), in spite of they used a higher concentration of topical bevacizumab.
Postoperative short-term therapy of topical bevacizumab 5 mg/mL in recurrent pterygium surgery by conjunctiva auto graft showed a lower recurrence rate but not statistically significant different than without its use, however it reduces significantly corneal neovascularization which may be not the main triggering factor in pterygium recurrence but it may be beneficial in other ocular situations.