Abstract: Primary vitreoretinal lymphoma (PVRL), as a subset of primary central nervous system lymphoma (PCNSL), is a rare and fatal ocular malignancy. Most PVRL masquerades as chronic posterior uveitis, which makes the clinical diagnosis challenging. Vitreous cells, subretinal lesions and imaging techniques are essential for clinical diagnosis. Importantly, cytopathology/histopathology identification of malignant cells is the gold standard for the diagnosis of PVRL. In addition, molecular detection of immunoglobulin heavy chain (IgH) or T cell receptor (TCR) gene rearrangements, immunophenotyping for cell markers, and cytokine analysis of interleukine-10 elevation are often used as adjunct procedures. Current management of PVRL involves local radiation, intravitreal chemotherapy (methotrexate and rituximab), with or without systemic chemotherapy depending on the involvement of non-ocular tissues. In cases with concomitant PCNSL, systemic high-dose methotrexate/rituximab based therapy in conjunction with local therapy, whole brain radiotherapy and/or autologous stem cell transplantation is considered. Although PVRL normally responds well to initial treatment, high rates of relapse and CNS involvement usually lead to poor prognosis and limited survival. A professional team of medical experts in ophthalmologists, ocular pathologists, neuro-oncologists and hemato-oncologists is essential for optimizing patient management.
In 1942, a case of lymphomatous processes as a type of stem cell lymphoma affecting the eyelid was published (1). Few more cases of lymphosarcoma/lymphoma were randomly reported later on; however, all of them only involved the eyelid and orbit (2). Until 1951, the first diagnosis of intraocular reticulum cell sarcoma was made in a 27-year-old Caucasian male; interestingly enough, the patient was diagnosed with testicular lymphosarcoma shortly after his enucleation (3). In 1968, for the first time, Vogel et al. reported six histological cases of intraocular reticulum cell sarcoma involving the retina (4). Since then, rare cases and case series of this intraocular malignancy have been presented worldwide (5-10). In the 1980s, the formerly called reticulum cell sarcoma was defined as a subtype of primary central nervous system lymphoma (PCNSL) and named primary intraocular lymphoma (PIOL), which represents any lymphomas originating from the ocular tissues (11). Later on, due to the distinctive features of lymphoma originating from the retina/vitreous and the choroid, “PVRL” is proposed to replace PIOL in 2009 (12,13).
To make PVRL more understood, it begins with one of the first clinically relevant lymphoma classification in general by Henry Rappaport in 1966. Based on cellular morphology, Rappaport classified lymphoma into Hodgkin’s disease, lymphosarcoma and reticulum cell sarcoma; and the early PVRL specifically belongs to the reticular cell sarcoma (14). In 1982, the National Cancer Institute, National Institutes of Health initiated the Working formulation to classify non-Hodgkin’s lymphoma and replaced the Rappaport system. In 1994, the International Lymphoma Study Group proposed the Revised European-American Classification of Lymphoid Neoplasms (REAL) (15), which provoked another round of lymphoma classification by the World Health Organization (WHO). As the leading system for lymphoma classification, the WHO classification of neoplasms of the hematopoietic and lymphoid tissues, published in 2001, updated in 2008 and revised in 2016, represents a worldwide consensus on the diagnosis of the neoplasms and is adopted by pathologists and clinicians (16-18). Diffuse large B-cell lymphoma (DLBCL), as the most common subtype of non-Hodgkin’s lymphoma, accounts for the majority of PVRL and PCNSL (13,18,19); whereas only a small percentage of PVRL is of T-cell or natural killer (NK)-cell origin. On the basis of gene expression profiling results, DLBCL can be further subdivided mainly into the germinal center B-cell-like (GCB) and activated B-cell-like (ABC) subgroups (18-20). Because the GCB and ABC subgroups differed in their somatic alterations, aberrant activation of intracellular signaling pathways, pharmacological activities and clinical outcomes (21,22), the revised 2016 WHO classi?cation requires the identi?cation of these two subtypes (18).
Intraocular lymphoma can be divided into primary (PVRL), uveal and secondary intraocular lymphomas, and intravascular lymphomatosis based on locations and clinical courses of the malignancy. When compared with the uveal and secondary intraocular lymphomas, PVRL has a much higher incidence affecting the retina than the uvea, and it can substantially involve the vitreous, retina, subretina, and optic nerve. Primary choroidal/uveal extranodal marginal zone lymphoma (EMZL) mucosa-associated lymphoid tissue (MALT) lymphoma, mainly involving the uveal tract, is less common and belongs to the low-grade B-cell lymphoma. The secondary intraocular lymphoma, refers to the metastasis from systemic lymphoma, is mainly hematogenous metastatic to the uvea, particularly the choroid, where has a rich blood supply. However, it may also metastasize to the retina, called systemic metastatic retinal lymphoma (SMRL). The diagnosis of SMRL is made when there is a clinical history of systemic lymphoma (particularly from nasopharynx, testis or skin), and lymphoma cells are identified in the vitreous and/or retina. Molecular analysis is more useful than vitreous cytokine measurement for SMRL diagnosis (23). Intravascular lymphomatosis is an extremely infrequent form of systemic extranodal non-Hodgkin’s lymphoma. It is characterized by the proliferation and aggregation of large B-lymphoma cells within the lumen of small blood vessels; only rare cases have been reported (24).
Because not all the lymphomas with vitreoretinal involvement represent PVRL, systemic evaluation as well as ophthalmic examination are of equal importance for accurate diagnosis. Salomao et al. conducted a retrospective review of 55 vitreous specimens from 55 patients to rule out malignancy in Mayo Clinic. Among the 13 specimens diagnosed with lymphoma, 3 (5%) of which had systemic lymphoma (2 DLBCL, 1 low-grade B-cell lymphoma and testicular lymphoma) without CNS involvement (25). Although systemic involvement in patients with vitreoretinal lymphoma is rare, the retrospective study has emphasized the importance of systemic evaluation in addition to CNS inspection in all patients presented with vitreoretinal lymphoma.
The case report introduces a comprehensive review with illustrations on clinical manifestation, diagnosis, management and prognosis of PVRL. A 49-year-old healthy Chinese female complained about visual loss, redness and pain in her right eye. At presentation at a local hospital on February 28th 2016, the visual acuity was 0.3 (20/67) in her right eye and 1.0 (20/20) in her left, the intraocular pressure (IOP) was within normal range in both eyes. Examination showed conjunctival injection, vitreous opacity and serous retinal detachment in the right eye. The left eye was reported unremarkable. The patient was diagnosed with Vogt-Koyanagi-Harada disease and treated with topical corticosteroids and oral cyclosporine A. On March 31st, in addition to decreased vision and ocular redness and pain, the patient had increased IOP of 45–55 mmHg and elevated subretinal lesions by sonography. Her cranial magnetic resonance imaging (MRI) was unremarkable. With further decreased vision, uncontrolled IOP and elevated subretinal lesions in her right eye, the patient was referred to Zhongshan Ophthalmic Center, Sun Yat-sen University in April 2016.
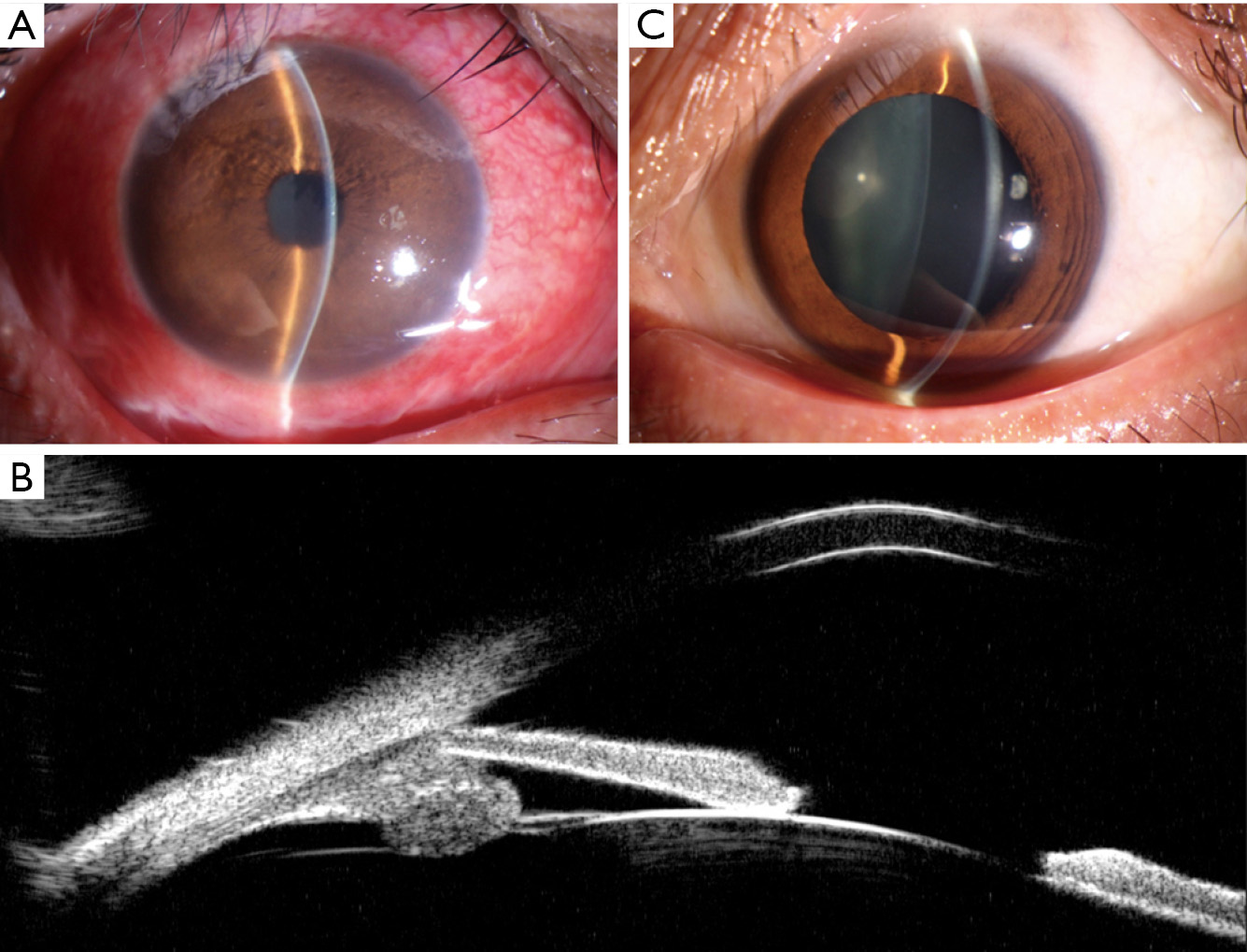
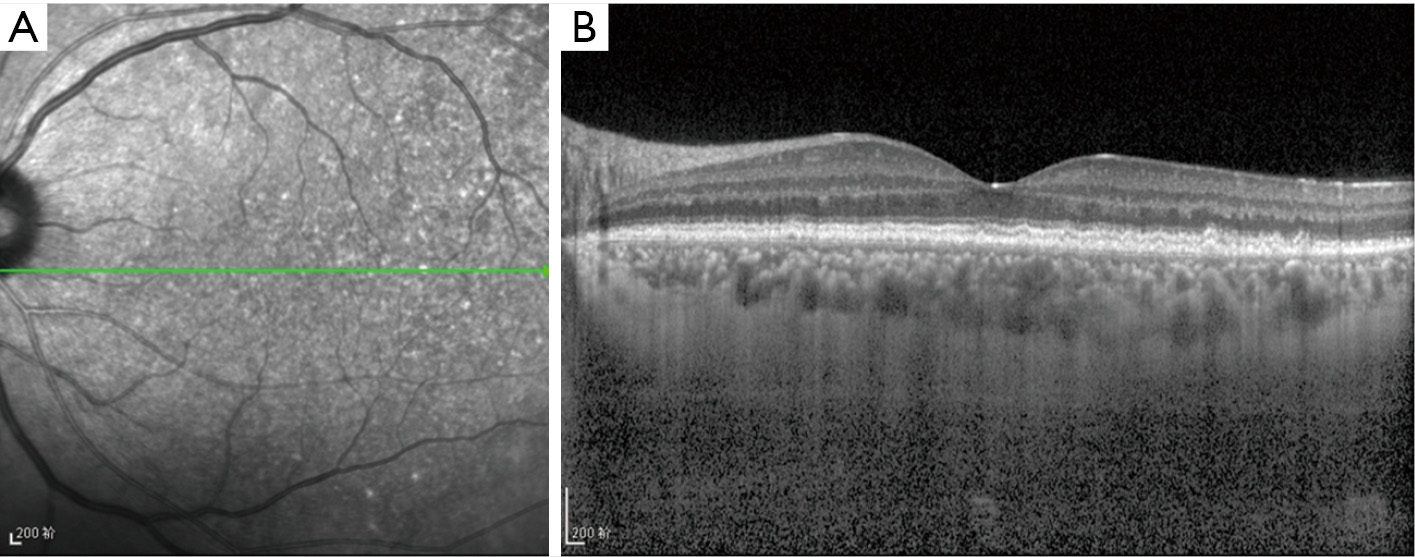
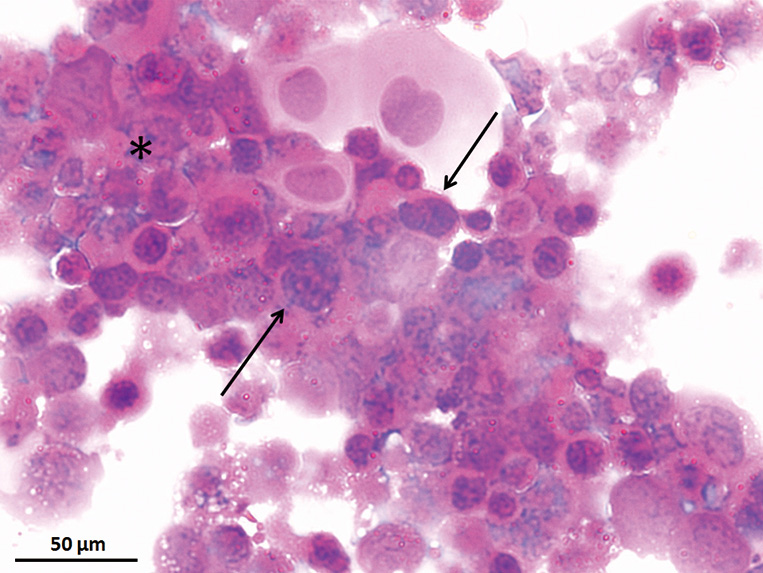
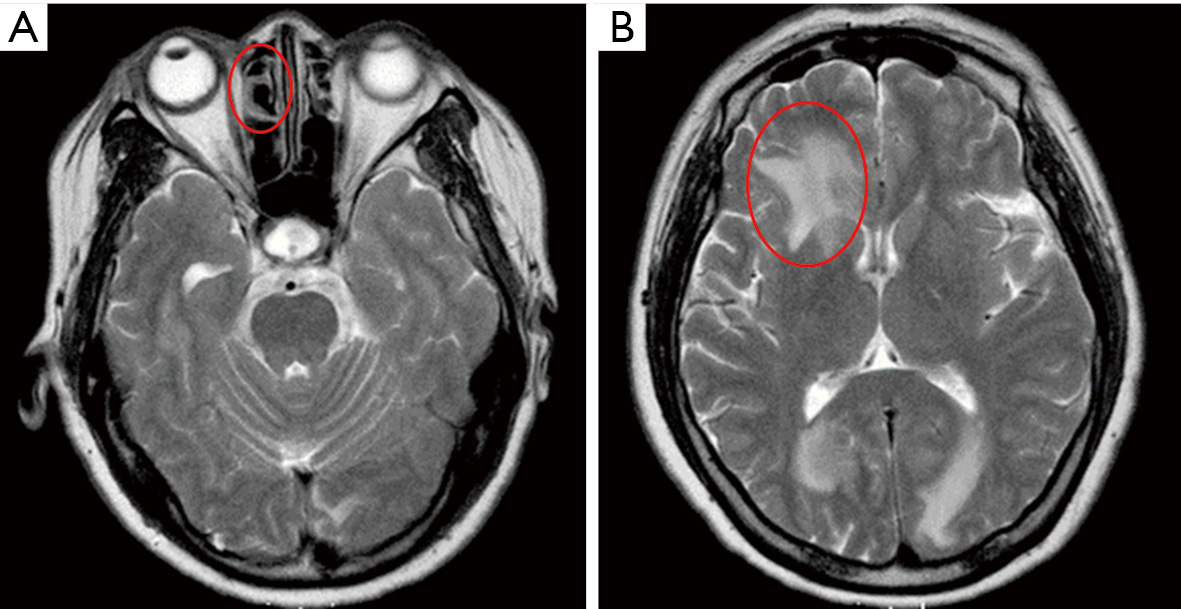
At presentation, her vision was 0.1 (20/200) in the right eye and 1.0 (20/20) in the left, the IOP was 50 mmHg in the right eye and 15 mmHg in the left. In the right eye, there was moderate conjunctival injection, mild corneal edema and constricted pupil with posterior synechiae (Figure 1A). The vitreous was moderately infiltrated and opaque. There was massive choroidal detachment with barely visible fundus. Ultrasound biomicroscopy showed anterior chamber angle closure (Figure 1B). Her left cornea, anterior chamber and pupil were unremarkable; minimal lens opacity was noted (Figure 1C). However, multiple small yellow subretinal spots were visible in the posterior pole (Figure 2A) and in the temporal periphery (Figure 2B). In the superior periphery, a large ill-defined, yellowish orange subretinal lesion was surrounded by multiple small infiltrates (Figure 2C). Fluorescein angiography (FA) showed mild leakage and retinal pigment epithelium (RPE) granularity in the middle phase (Figure 2D). Large areas of hypofluorescence and multiple hyperfluorescence at the level of the RPE were noted in the superior periphery (Figure 2E), corresponding to the subretinal yellow lesions noted on funduscopy. At the same lesional area, indocyanine green angiography showed large ill-defined hypofluorescent lesions at the late phase (Figure 2F). Optic coherence tomography (OCT) revealed mild irregularity of the inner/outer segments of photoreceptors and RPE/Bruch’s membrane complex (Figure 3). The patient was continuously treated with anti-inflammatory and anti-glaucoma agents but showed no improvement in both eyes. In May 2016, the vision of the right eye decreased to light perception. A diagnostic vitrectomy was performed in her right eye. Cytopathology of the vitreous specimen revealed large atypical lymphoid cells (Figure 4). Meanwhile, T2W MRI of the brain showed right ethmoid sinus enlargement (Figure 5A) and thickening in the axial section; a sinus biopsy confirmed its origin as DLBCL. A brain lesion in right corpus callosum was also revealed in the T2W MRI scans (Figure 5B). The patient remains asymptomatic in the left eye and is undergoing local and systemic chemotherapy for her PVRL/PCNSL.
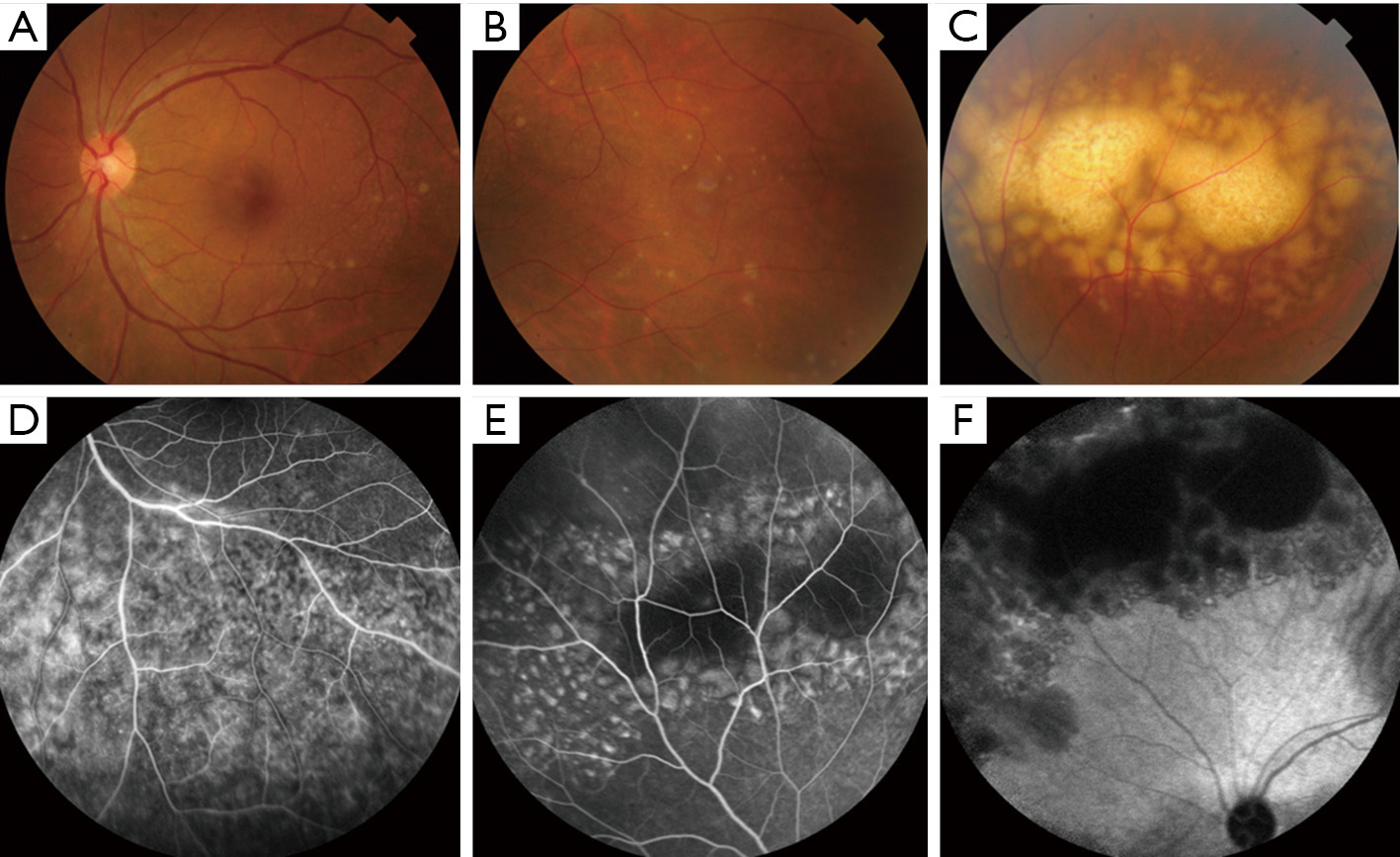
As a subtype of PCNSL, PVRL typically occurs in older individuals with a median age of 60 years (13,26). It usually masquerades as posterior uveitis but does not respond well to anti-inflammatory therapy (13,27). Most PVRL presents with vitreous haze and retinal/subretinal infiltrations. The vitreous haze and marked vitreous cells in sheets and/or clumps are typical features of PVRL (27). Although with moderate to severe vitreous opacity, vision is often better than expected for the degree of vitreous cellularity. The most characteristic feature of PVRL is the yellowish white/orange subretinal infiltrates that may enlarge and coalesce over time (Figure 2A,B,C) (27). Moreover, other abnormalities like hemorrhages, retinal vasculitis, optic nerve infiltrates, and/or serous retinal detachment can occur (6,28). In addition to the posterior segment involvement, some patients initially develop some anterior segment manifestations, including corneal edema, hypopyon, pupil distortion and/or iritis (29). Clinical manifestations and ocular imaging, including fundus autofluorescence, FA and OCT can often provide characteristic features of PVRL.
Fundus autofluorescence is useful for detecting early RPE abnormality. The coexistence of PVRL cells at or above the RPE presents with multiple weak or bright hyperfluorescent spots (Figure 3A). The hypofluorescent areas highlight the PVRL cells above the RPE or RPE atrophy. FA is helpful to determine the extent of PVRL infiltration and degree of RPE atrophy (Figure 2D,E). Hyperfluorescent window defects and round hypofluorescent lesions are the most common findings in PVRL (30,31). Clusters of round hyper or hypofluorescent lesions about 100 μm in size at early and late phases can be found (32). RPE disturbances with granularity, blockage and late staining are commonly shown (30,31). Heterogenous choroidal fluorescence can also occur in some PVRL patients (33). Indocyanine green angiography is not as helpful as FA; however, it sometimes shows small round hypofluorescent lesions disappear at the late phase in PVRL (Figure 2F) (33). OCT often reveal hyperreflective nodules in the outer retina and disruption of the ellipsoid zone in approximately half of the PVRL patients (Figure 3B) (34). These nodules presented as subretinal infiltrates are confirmed to be the PVRL deposits located between the RPE and Bruch’s membrane by pathologic studies (32). OCT may allow for early detection of small macular abnormalities and aid in monitoring of treatment efficacy in this disease (34).
To facilitate differential diagnosis or rule out involvement of CNS or other systemic, systemic evaluation such as brain computerized tomography (CT)/MRI scans (Figure 5), bone marrow biopsy, testicular ultrasonography, whole-body positron emission tomography (PET) or PET-CT scanning has been recommended in consideration of situations of each PVRL individual.
PVRL diagnosis requires clinical suspicions and related adjunct examinations. Among all the tests, cytopathology/histopathology is the gold standard for PVRL diagnosis. Clinical samples collected from the cerebrospinal fluid (CSF), aqueous, vitreous, subretinal fluid and/or chorioretinal tissue are eligible for histopathology. Among these specimen options, the vitreous fluid from diagnostic vitrectomy is mostly preferred (13,35). Other external or transvitreal surgeries for retinal, subretinal or chorioretinal biopsies are also considered clinically, especially when no optimal cells are yielded from the diagnostic vitrectomy specimens. Cytopathology provides direct evidence of the featured large atypical lymphoid cells with large irregular nuclei, prominent nucleoli and scanty basophilic cytoplasm (Figure 4) (27). However, the atypical lymphoma cells in the specimens usually admix with reactive lymphocytes, degenerative/necrotic cells and debris, which makes the diagnosis quite difficult. Additionally, the paucity and fragility of the atypical lymphoid cells further challenge the cytopathology diagnosis (36). Thus, all the biopsy specimens require appropriate handling and prompt processing; moreover, expert cytopathologist and/or ophthalmic pathologist are required for the tissue diagnosis.
Although cytological analysis is critical for identifying tumor cells in PVRL, further classification of the lymphoma cell origin must rely on immunophenotyping by immunohistochemistry/flow cytometry and molecular evaluation with polymerase chain reaction (PCR). Monoclonality is the result of the unique proliferation mechanism in B- and T-cell lymphomas, which allows immunohistochemistry staining of the B cell markers (e.g., CD19, CD20, CD22 and restricted lambda and kappa chains) and T cell markers (e.g., CD3 and CD30). Recently, flow cytometric immunophenotyping has been more often used as an alternative to conventional staining. Both immunohistochemistry staining and flow cytometry are useful in the classification of PVRL (37,38).
During the normal B cell development, immunoglobulin genes undergo a complex rearrangement process to produce diverse antibody coding sequences. Thus, polyclonal populations of B cells harbor polyclonal immunoglobulin heavy chain (IgH) gene rearrangements. In contrast, neoplastic B cell population in PVRL derives from a single cell, which allows each tumor cell to own the same unique rearrangement (39-42). Complementarity-determining region 3 (CDR3) of the IgH gene is the most variable region of rearrangement in B-cell lymphoma (41,43,44). Similarly, T-cell receptor (TCR) gene rearrangement, most commonly TCR gamma region, serves as a molecular marker of clonal expansion in T-cell lymphoma. Therefore, the monoclonal rearrangement of IgH genes in B-cell lymphomas and TCR genes in T-cell lymphoma is a feature of PVRL, which implements the molecular diagnosis and further classification of tumor origin using PCR. Importantly, to make the molecular diagnosis accurate and specific, microdissection of the atypical lymphoma cells is an initial step to detect gene rearrangements in PVRL cells (44,45). Without microdissection, molecular diagnosis may result in false negative results.
Cytokine analysis from the CSF or intraocular fluids can show characteristic elevation of several cytokines in PVRL. Clinically, specimens of diluted or undiluted vitreous obtained by vitreous aspiration or pars plana vitrectomy have been the mainstay in PVRL diagnosis. Interleukin-10 (IL-10) is first known as a growth and differentiation factor for B lymphocytes. Not surprisingly, B-lymphoma cells produce abundant IL-10 in PVRL. On the contrary, IL-6 is produced by a wide variety of cells including inflammatory cells and certain ocular resident cells and regarded as a marker of pro-inflammatory diseases (46). As an internal control for the diluted vitreous samples, IL-6 is simultaneously measured in the same specimen with IL-10. Previous studies have demonstrated that elevated IL-10 levels with an IL-10 to IL-6 ratio greater than 1.0 are the most useful predictor of PVRL (47-49). Wolf et al. analyzed vitreous samples from 35 PVRL and 64 uveitis patients. For the PVRL group, the geometric mean IL-10 to IL-6 ratio was 5.23 in the PVRL group; while the ratio for the uveitis group was 0.23. Correct classification by the ratio at cutoff rule greater than 1.0 was 74.7% of the time, with a sensitivity of 0.74 and a specificity of 0.75 (49). Cassoux et al. compared both aqueous and vitreous specimens in 51 PVRL and 108 uveitis patients (74 with proven etiology and 34 with idiopathic uveitis). At the cutoff of 50 pg/mL, aqueous specimen had a sensitivity of 0.89 and a specificity of 0.93; comparatively, a cutoff value of 400 pg/mL yielded a specificity of 0.99 and a sensitivity of 0.80 in the vitreous. These results have demonstrated that measurement of IL-10 in the aqueous is a good screening test to reduce diagnostic delays (50). In another study of 30 patients (22 PVRL and 8 viral retinitis) in Japan, both IL-10 and IL-6 levels in the vitreous were measured. The mean concentrations of IL-10 and IL-6 in the 30 vitreous samples of the 22 PVRL patients were 4,187±1,696 and 617±377 pg/mL, respectively. In contrast, the mean concentrations of IL-10 and IL-6 in the 10 vitreous controls were 181±75 and 6,705±3,053 pg/mL, respectively. Eighteen of the 22 (81.8%) PVRL suspects met the criteria of PVRL diagnosis as IL-10 concentration above 100 pg/mL and IL-10/IL-6 greater than 1.0. On the other hand, none of the 10 control patients met the criteria (51). Fisson et al. analyzed the helper T-cell type 1 (Th1)/Th2 cytokine in PVRL and uveitis patients. Besides elevation of IL-10 in PVRL patients, they found that the combination of IL-10/IL-6 and IL-10/interferon-gamma (IFNγ) ratios was beneficial for differentiating PVRL from uveitis as well as for therapeutic follow-up of PVRL (52). These cytokine combinations would further increase the diagnostic value of cytokine evaluation for PVRL diagnosis.
In additional to the elevation of IL-10 protein in PVRL patients, mutations in the IL-10 gene has been detected in PVRL. By evaluating 27 PVRL, 59 PCNSL and 98 normal controls, Ramkumar et al. demonstrated that the IL-10-1082 A allele was significantly associated with upregulated IL-10 transcript levels in PCNSL as well as higher IL-10/IL-6 ratios in vitreous of PVRL. Because higher IL-10 levels are correlated with more aggressive disease in both PVRL and PCNSL, this finding can be referred as an important and potentially clinically significant observation (53). Tuo et al. screened 168 human mature microRNAs in the vitreous specimens of 17 PVRL and 12 uveitis patients and found that microRNA-155 was significantly higher in the vitreous of uveitis patients than PVRL, which may be helpful in the differential diagnosis of PVRL from uveitis (54).
We collected 227 specimens from 214 patients with masquerade syndrome and compared the diagnostic values among cytology, molecular analysis and cytokine levels. Overall, the molecular technique has the highest diagnostic value (0.995) in PVRL compared with cytology (0.89) and cytokine levels (0.875). Cytology has higher specificity (0.99) and positive predictive value (0.99) than cytokine analysis; but cytokine analysis has higher sensitivity (0.88) than cytology in PVRL (55). As one single diagnostic technique cannot guarantee the precise diagnosis of PVRL, multiple diagnostic techniques are usually recommended. Three panels of experts from British Neuro-Oncology Society, International PCNSL Collaborative Group for PVRL, and National Comprehensive Cancer Network (USA) consensually recommend cytology, immunolabeling and gene rearrangement detection as diagnostic methods for PVRL (26).
As a rare intraocular malignancy, a large group of intraocular diseases are candidates of differential diagnosis. These entities include but are not limited to inflammatory disorders (e.g., posterior or intermediate uveitis or panuveitis, multifocal choroiditis, Behcet disease), infectious causes (e.g., tuberculoma, cytomegalovirus retinitis, endophthalmitis) or other neoplasms (e.g., metastatic cancers, amelanotic melanoma). Meanwhile, as a subset of PCNSL, diagnosis of PVRL requires a strategic workflow to achieve accurate and prompt diagnosis. For PVRL suspects, a brain MRI and lumbar puncture can first screen the CSF involvement. PCNSL is diagnosed when CSF is positive for lymphoma. If CSF screen is negative, intraocular fluid sampling (such as aqueous tap or diagnostic vitrectomy) with proper specimen handling is considered. Similarly, PVRL diagnosis is confirmed with positive results. However, for negative specimens, repeated aqueous or vitreous sampling are recommended. Additionally, in patients with repeatedly negative results but highly suspected for PVRL, retinal/subretinal tissue biopsy or enucleation may be considered if these procedures are needed or diagnostic vitrectomy was failed for these patients (23,25,43,56,57).
No standard therapy has been made due to the rarity of PVRL. Like other malignancies, PVRL treatment may require single or a combination of radiation, local/systemic chemotherapy and autologous stem cell transplantation (ASCT). In 2011, the International PCNSL Collaborative Group issued a set of therapeutic principles for PVRL as an optimal therapy. Systemic therapy is required if disease involves the CNS and local therapy is used if disease only involves the eye; patients should be carefully and frequently followed; and the close collaboration should be established between neuro-oncologists and ophthalmologists (32).
PVRL patients with only one eye involved can be treated with local ocular therapy, including external-beam radiotherapy (EBRT) and intravitreal chemotherapy of methotrexate and/or rituximab (32,58). The typical EBRT is highly effective to control primary lesions, involving a total of 30–45 Gy delivered in 15–25 fractions (59-61). Due to the risks of irreversible vision loss caused by radiation retinopathy or optic atrophy, EBRT cannot be routinely repeated in recurrent PVRL (58). However, for patients with bilateral intraocular involvement or those who cannot tolerate intravitreal chemotherapy, EBRT remains an initial method.
Because the blood-ocular barriers limit the penetration of systemic chemotherapeutic agents into the inner eye, intravitreal injection serves as a better option with good response and low recurrence rates. Intravitreal methotrexate has been regarded as a good first-line treatment option for PVRL due to its prolonged tumoricidal concentration and longer-lasting effects than systemic administration (62). At a dose of 0.4 mg/0.1 mL, intravitreal methotrexate has been shown to control tumor in naive and relapsed PVRL; it can also be applied in combination with intravitreal rituximab, systemic chemotherapy or EBRT in PVRL/PCNSL patients (62-65). Frenkel et al. treated 44 eyes of 26 PVRL patients, with the intravitreal methotrexate twice weekly for 4 weeks, once weekly for 8 weeks and then once monthly for 9 months, for a total of 25 injections. In their 10-year evaluation, clinical remission was reached after 6.4 doses of intravitreal methotrexate; 95% of the involved eyes needed 13 injections or less to be cleared of tumor cells. The most common side effects of intravitreal methotrexate was corneal epitheliopathy, which usually appeared after the third injection and began to subside when the intervals between injections increased (65,66). No corneal endothelial damage has been noted by specular microscopy in 6-month repeated injection (67). Some cases of resistance to intravitreal methotrexate were documented, which may be due to the decreased intracellular accumulation (68,69).
Intravitreal methotrexate combined with systemic high-dose methotrexate is proven to be effective in PVRL patients (70,71). Akiyama et al. reviewed 10 treatment-naive PVRL patients, who were treated with weekly intravitreal methotrexate until the ocular lesions were resolved, followed by five cycles of systemic high-dose methotrexate (3.5 g/m2) every other week. All patients achieved complete response for their ocular lesions with rapid decrease of intravitreal IL-10. Four of 10 patients experienced the CNS lymphoma development and the mean PCNSL-free survival time was 58.3 months in a median follow-up of 29.5 months, whereas the 2-year survival was 37.5 months in the single intravitreal methotrexate (70).
Rituximab is a genetically engineered human monoclonal antibody directed against the B-lymphocyte specific antigen CD20 that is expressed only by hematopoietic and mature B cells (72,73). Rituximab was approved in 1997 to treat B-cell lymphoma and leukemia, and it has been shown to improve the outcome in PCNSL patients and reduce the CNS relapse rate in high-risk patients (74). Because of its poor leptomeningeal compartment penetration, intrathecal administration of rituximab is preferred and can be combined with local/systemic methotrexate or EBRT for PCNSL. Given that the vast majority of PVRL/PCNSL is CD20 positive DLBCL, rituximab is being used increasingly as local chemotherapy for PVRL. Without evidence of intraocular toxicity at 1 mg/0.1 mL (75), individual local injection of rituximab is potentially effective for PVRL. Hashida et al. reported weekly intravitreal injections of rituximab on 20 eyes of 13 females with PVRL for 4 weeks; although it was efficacious as alternative treatment, the anterior segment lesions recurred in 11 (55%) eyes of 9 patients and resolved with another course of injections. Common adverse effects of intravitreal rituximab involve transient IOP elevation and iridocyclitis (76).
Due to a half rate of relapse when use individually, rituximab is more often used in combination with other local or systemic chemotherapeutics. Rituximab with high-dose methotrexate was added to the institutional standard protocol for patients with newly diagnosed PCNSL at the Johns Hopkins University (77). The combination of rituximab with high-dose methotrexate may improve the complete remission rates as well as the overall and progression-free survivals in immunocompetent patients with newly diagnosed PCNSL (78). Rituximab-based intravitreal chemotherapy has been reported to be safe and effective to induce remission in a majority of eyes with vitreoretinal lymphoma (79-81). Larkin et al. treated 48 eyes of 34 vitreoretinal lymphoma patients with a median of 3.5 intravitreal injections of rituximab for new diagnosis (68.8%), progressive disease (29.9%) and maintenance therapy (2.1%). Intravitreal rituximab with methotrexate was the sole treatment in 19 eyes (39.6%). Among the 48 eyes, 31 eyes achieved complete remission after a median of three injections, 7 developed recurrent disease; and 11 achieved partial remission. Although rituximab-associated complications reported in 25% of the eyes, a 2-line loss of Snellen visual acuity occurred in only 2 eyes (4.2%) (81). This result is encouraging.
In cases of bilateral PVRL without PCNSL involvement, there is still a recommendation towards local chemotherapy, although systemic treatment should be considered (32,58). Besides the high-dose methotrexate based therapy, CHOP regimens (cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab are choices for DLBCL. Currently, there is no consensus on whether to choose systemic therapy in PVRL patients without CNS involvement. In a series of patients with isolated PVRL, the use of systemic chemotherapy was not proven to prevent CNS involvement and was associated with more severe adverse effects compared with local treatment (82). The development of CNS manifestations after PVRL was reported similar between PVRL patients receiving local ocular treatments and patients without systemic therapy. However, some studies have showed that the preventive systemic chemotherapy, especially high-dose methotrexate, significantly prolonged the time to the onset of CNS involvement when compared with local ocular therapy (83). Thus, the potential benefit of systemic chemotherapy need to be further studied and carefully considered in terms of more severe adverse effects compared with local therapy (82).
For PVRL patients with CNS involvement, high-dose methotrexate based therapy (possibly with systemic rituximab) in conjunction with local ocular therapy is considered. Whole brain radiotherapy in conjunction with ocular radiotherapy is recommended in those who have failed systemic therapy and are too debilitated or do not meet criteria for more aggressive therapy such as ASCT (32). However, some neuro-oncologists may not give local treatment to the eyes when there is concurrent ocular and CNS lymphoma (13).
During the initiation of B-cell receptor (BCR) complex signaling cascades, Bruton’s tyrosine kinase (BTK) is recruited to the cell membrane and activates other kinases (84). This subsequently leads to an increase in intracellular Ca2+ and activation of nuclear factor-κB (NF-κB), which are involved in many cellular activities such as cell proliferation, survival, differentiation and apoptosis (85). In addition, BTK plays a role in chemokine receptor and integrin signalings and is essential to chemokine-mediated homing and adhesion of B cells (86). Davis et al. have shown that activated BCR signaling is required for cell survival in the ABC subtype of DLBCL (84). As a critical effector molecule in the BCR signaling pathway that is essential for the survival and proliferation of malignant B cells, BTK inhibitors have been investigated as potential treatments. Ibrutinib is an orally-administered and selective inhibitor of the BTK (86). It forms a covalent bond with a cysteine residue-Cys481 located at the rim of the ATP-binding pocket in BTK. Ibrutinib effectively inhibits cell cycle and proliferation, target BCR-controlled and chemokine-controlled-integrin-mediated adhesion, which results in lymphoma regression due to mobilization of the malignant cells from their protective niches into the circulation (87). Because BTK is expressed in non-T cell hematopoietic cell lineages, no toxic effects are exerted on T cells. The safety profile of ibrutinib is likely further enhanced by the once-daily administration and rapid absorption and elimination, with a half-time of 2–3 hours (88). Such intermittent exposure limits the duration of off-target effects. Additionally, ibrutinib did not reduce serum immunoglobulin levels, even in patients with prolonged treatments (88). Wilson et al. have reported that 38 cases of ABC lymphoma with BCR mutations responded to ibrutinib frequently in more than half of the patients, especially those with concomitant myeloid differentiation primary response 88 (MYD88) mutations (89). MyD88 is a universal adapter protein as it is used by almost all toll-like receptors (TLRs, except TLR3) to activate the transcription factor NF-κB. Except MyD88 mutation in ABC subtype of DLBCL, high frequency of MyD88 mutations is recently reported in PVRL. Bonzheim analyzed vitreous specimens of 29 PVRL patients and found MYD88L265P mutation existed in 20 cases (90). Apparently, ibrutinib would become a promising agent for PVRL that typically belongs to DLBCL with a high incidence of MYD88 mutation.
The rationale for ASCT in PCNSL is to achieve therapeutic drug concentration in the CNS and to overcome chemotherapy resistance (91,92). ASCT has shown be feasible and highly effective in patients with PCNSL (92,93). During the past 15 years, ASCT has been included as a part for dose-intensive chemotherapeutic consolidation (91,94). Langner-Lemercier et al. studies 563 PCNSL patients treated with first-line therapy and 256 of them with relapsed or refractory disease. The patients who received salvage therapy followed by consolidation therapy (mostly intensive chemotherapy plus ASCT) experienced prolonged survival compared with those who did not receive salvage or consolidation therapy. Conversely, patients experiencing late relapses and/or undergoing consolidation with intensive chemotherapy plus ASCT experienced prolonged survivals (95).
Overall, PVRL has a poor prognosis with lower rates of over and progression-free survivals. However, single site involvement of either eye or CNS has a better prognosis than the double site involvement (PVRL and PCNSL) (71,96). The 5-year survival rate is higher in patients with isolated PVRL than in those with CNS involvement (71,82). However, the duration of complete remission did not differ between patients with and without CNS involvement (71). Kreher reported that the median progression-free survival in the PCNSL patients with intraocular involvement (3.5 months) was shorter than the ones without PVRL (8.3 months) (96). That is, intraocular involvement at diagnosis of PCNSL was an independent negative prognostic indicator for the progression-free survival and overall survival (96). Local ocular therapy could improve disease control in PCNSL patients but may have no effect on the overall survival. Grimm et al. retrospectively studies 221 PCNSL patients from 16 centers in seven countries. The median progression-free survival was prolonged in patients who received dedicated ocular therapy at 19 months as compared to 15 months in those who did not receive dedicated ocular therapy. However, the initial treatment approach did not impact the overall survival (97).
PVRL is a rare ocular malignancy, with an incidence of approximately one in a million in the United States. However, 80% of the patients with PVRL further develop PCNSL, which is a fatal disease. As the most common malignancy masquerading as posterior uveitis, PVRL diagnosis requires clinical history, fundus examination, imaging and histopathology. In addition, other adjunct methods, such as immunohistochemistry, flow cytometry, cytokine measurement and molecular analysis, are highly recommended. A team of ophthalmologists, ocular pathologists, neuro-oncologists and hemato-oncologists should be organized for optimizing patient management.