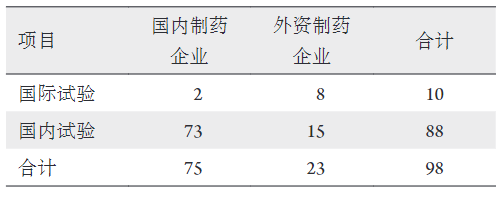
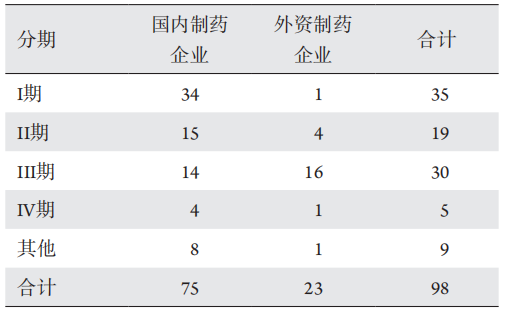
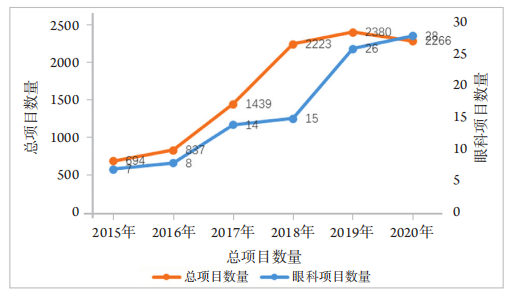
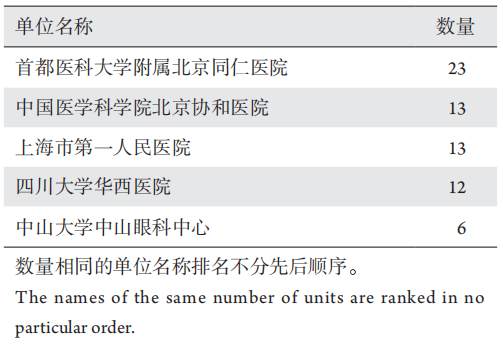
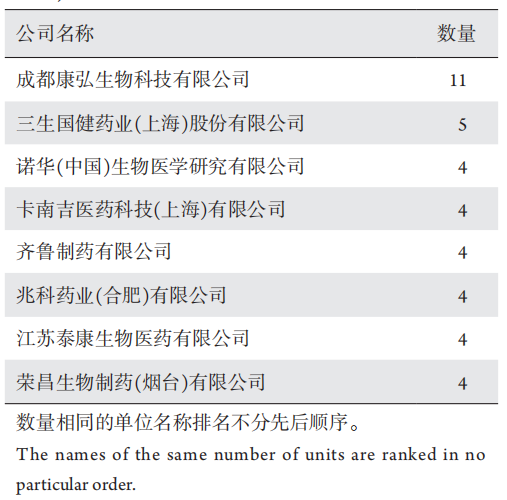
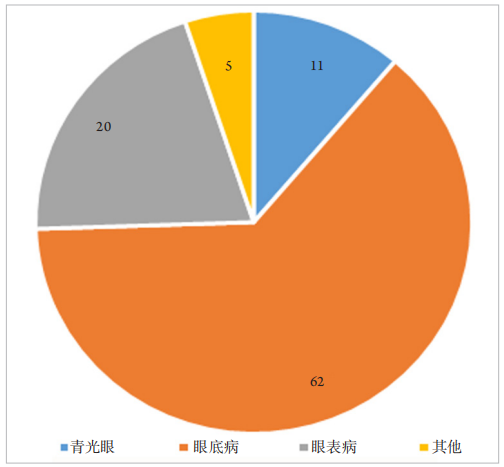
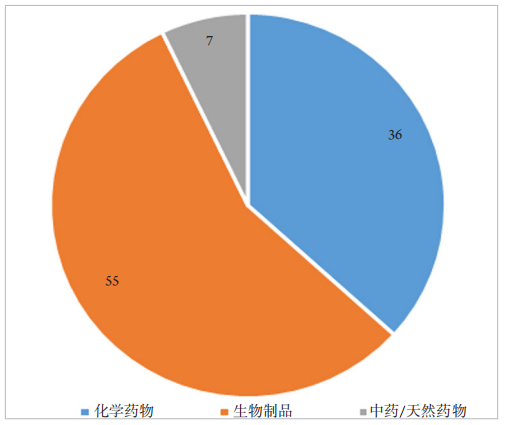
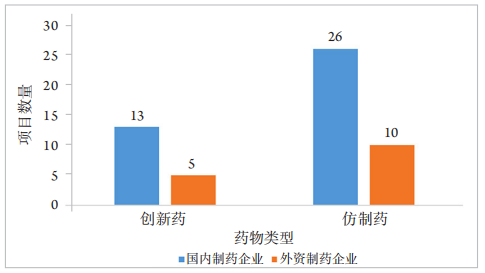
1、王若男, 钱仪敏, 宋硕, 等. 眼底疾病治疗药物非临床研究策略[ J]. 中国新药杂志, 2020, 29(15): 1723-1728.
WANG Ruonan, QIAN Yimin, SONG Shuo, et al. Non-clinical research strategy for the treatment of fundus diseases[ J].Chinese Journal of New Drugs, 2020, 29(15): 1723-1728.王若男, 钱仪敏, 宋硕, 等. 眼底疾病治疗药物非临床研究策略[ J]. 中国新药杂志, 2020, 29(15): 1723-1728.
WANG Ruonan, QIAN Yimin, SONG Shuo, et al. Non-clinical research strategy for the treatment of fundus diseases[ J].Chinese Journal of New Drugs, 2020, 29(15): 1723-1728.
2、新领先. 眼科药市场重新洗牌 竞争格局正在重塑[N]. 中国医药报, 2020-07-24(004).
Xin Lingxian. Ophthalmic drug market reshuffle competition pattern is reshaping[N]. China Medical Journal. 2020-07-24(004).新领先. 眼科药市场重新洗牌 竞争格局正在重塑[N]. 中国医药报, 2020-07-24(004).
Xin Lingxian. Ophthalmic drug market reshuffle competition pattern is reshaping[N]. China Medical Journal. 2020-07-24(004).
3、张秀兰, 陈翔, 陈倩韵, 等. 中国眼科临床研究面临的机遇和挑战[ J]. 眼科学报, 2020, 35(5): 289-295.
ZHANG Xiulan, CHEN Xiang, CHEN Qianyun, et al. Opportunity and challenge of ophthalmic clinical researches in China[ J]. Yan Ke Xue Bao, 2020, 35(5): 289-295.张秀兰, 陈翔, 陈倩韵, 等. 中国眼科临床研究面临的机遇和挑战[ J]. 眼科学报, 2020, 35(5): 289-295.
ZHANG Xiulan, CHEN Xiang, CHEN Qianyun, et al. Opportunity and challenge of ophthalmic clinical researches in China[ J]. Yan Ke Xue Bao, 2020, 35(5): 289-295.
4、Yao C. Clinical trial in China: The status and challenge of data management and statistical analysis[ J]. J Evid Based Med, 2018, 11(1): 3-6.Yao C. Clinical trial in China: The status and challenge of data management and statistical analysis[ J]. J Evid Based Med, 2018, 11(1): 3-6.
5、东吴证券. 眼 科:黄金赛道中国崛起[ J]. 股市动态分析, 2020(18): 54.
Dongwu Securities. Ophthalmology: The rise of China on the golden Track[ J]. Stock Market Trend Analysis Weekly, 2020(18): 54.东吴证券. 眼 科:黄金赛道中国崛起[ J]. 股市动态分析, 2020(18): 54.
Dongwu Securities. Ophthalmology: The rise of China on the golden Track[ J]. Stock Market Trend Analysis Weekly, 2020(18): 54.
6、国务院关于改革药品医疗器械审评审批制度的意见[ J]. 中国药物评价, 2016, 33(2): 65-67.
Opinions of The State Council on reforming the examination and approval system of drugs and medical devices[ J]. Chinese Journal of Drug Evaluation, 2016, 33(2): 65-67.国务院关于改革药品医疗器械审评审批制度的意见[ J]. 中国药物评价, 2016, 33(2): 65-67.
Opinions of The State Council on reforming the examination and approval system of drugs and medical devices[ J]. Chinese Journal of Drug Evaluation, 2016, 33(2): 65-67.
7、中共中央办公厅. 国务院办公厅印发《关于深化审评审批制度改革鼓励药品医疗器械创新的意见》[ J]. 中国食品药品监管, 2017(10): 9-13.
General Office of the CPC Central Committee. General Office of the State Council issues Opinions on Deepening reform of The Review and Approval System and Encouraging Innovation of Pharmaceutical and Medical Devices. China Food Drug Administration, 2017(10): 9-13.中共中央办公厅. 国务院办公厅印发《关于深化审评审批制度改革鼓励药品医疗器械创新的意见》[ J]. 中国食品药品监管, 2017(10): 9-13.
General Office of the CPC Central Committee. General Office of the State Council issues Opinions on Deepening reform of The Review and Approval System and Encouraging Innovation of Pharmaceutical and Medical Devices. China Food Drug Administration, 2017(10): 9-13.
8、黄慧瑶, 吴大维, 王海学, 等. 2019年中国肿瘤药物临床试验进展[ J]. 中华肿瘤杂志, 2020, 42(2): 127-128.
HUANG Huiyao, WU Dawei, WANG Haixue, et al. Progress on clinical trials of cancer drugs in China, 2019[ J]. Chinese Journal of Oncology, 2020, 42(2): 127-128.黄慧瑶, 吴大维, 王海学, 等. 2019年中国肿瘤药物临床试验进展[ J]. 中华肿瘤杂志, 2020, 42(2): 127-128.
HUANG Huiyao, WU Dawei, WANG Haixue, et al. Progress on clinical trials of cancer drugs in China, 2019[ J]. Chinese Journal of Oncology, 2020, 42(2): 127-128.
9、梅隆, 李飒, 甄健存. 治疗黄斑变性眼用药物制剂进展[ J]. 临床药物治疗杂志, 2019, 17(6): 61-65.
MEI Long, LI Sa, ZHEN Jiancun. Progress of ophthalmic preparations for treating macular degeneration[ J]. Clinical Medication Journal, 2019, 17(6): 61-65.梅隆, 李飒, 甄健存. 治疗黄斑变性眼用药物制剂进展[ J]. 临床药物治疗杂志, 2019, 17(6): 61-65.
MEI Long, LI Sa, ZHEN Jiancun. Progress of ophthalmic preparations for treating macular degeneration[ J]. Clinical Medication Journal, 2019, 17(6): 61-65.
10、彭立, 谢青, 陈敏华. 抗VEGF治疗在眼科的临床意义及研究进展[ J]. 国际眼科杂志, 2020, 20(2): 282-285.
PENG Li, XIE Qing, CHEN Minhua. Clinical significance and recent research advances of anti-VEGF treatment in ophthalmology[ J]. International Eye Science, 2020, 20(2): 282-285.彭立, 谢青, 陈敏华. 抗VEGF治疗在眼科的临床意义及研究进展[ J]. 国际眼科杂志, 2020, 20(2): 282-285.
PENG Li, XIE Qing, CHEN Minhua. Clinical significance and recent research advances of anti-VEGF treatment in ophthalmology[ J]. International Eye Science, 2020, 20(2): 282-285.
11、全球眼科疾病抗体药物发展现状及趋势[N]. 新浪医药新闻. 2019-05-20.
Global ophthalmic disease antibody drug development status and trend[N]. Sina Medical News. 2019-05-20.全球眼科疾病抗体药物发展现状及趋势[N]. 新浪医药新闻. 2019-05-20.
Global ophthalmic disease antibody drug development status and trend[N]. Sina Medical News. 2019-05-20.
12、肖婷婷, 石珂, 缪振忠, 等. 临床抗青光眼药物的新进展[ J]. 眼科新进展, 2019, 39(2): 192-196.
XIAO Tingting, SHI Ke, MIAO Zhenzhong, et al. Novel advances in clinical on anti-glaucoma drug[ J]. Recent Advances in Ophthalmology, 2019, 39(2): 192-196.肖婷婷, 石珂, 缪振忠, 等. 临床抗青光眼药物的新进展[ J]. 眼科新进展, 2019, 39(2): 192-196.
XIAO Tingting, SHI Ke, MIAO Zhenzhong, et al. Novel advances in clinical on anti-glaucoma drug[ J]. Recent Advances in Ophthalmology, 2019, 39(2): 192-196.
13、黄鑫玲, 陈向东, 宋焰. 干眼的中西医治疗新进展[ J]. 中国中医眼科杂志, 2020, 30(6): 443-446.
HUANG Xinling, CHEN Xiangdong, SONG Yan. Advanced progress of Traditional Chinese Medicine and Western Medicine in treating dry eye[ J]. Chinese Journal of Ophthalmology, 2020, 30(6): 443-446.黄鑫玲, 陈向东, 宋焰. 干眼的中西医治疗新进展[ J]. 中国中医眼科杂志, 2020, 30(6): 443-446.
HUANG Xinling, CHEN Xiangdong, SONG Yan. Advanced progress of Traditional Chinese Medicine and Western Medicine in treating dry eye[ J]. Chinese Journal of Ophthalmology, 2020, 30(6): 443-446.
14、毕双双, 马雪峰, 戴馨. 阿托品对儿童及青少年近视发展的影响[ J]. 菏泽医学专科学校学报, 2021, 33(2): 33-34.
BI Shuangshuang, MA Xuefeng, DAI Xin. The influence of atropine on the development of myopia in children and adolescents[ J]. Journal of Heze Medical College, 2021, 33(2): 33-34.毕双双, 马雪峰, 戴馨. 阿托品对儿童及青少年近视发展的影响[ J]. 菏泽医学专科学校学报, 2021, 33(2): 33-34.
BI Shuangshuang, MA Xuefeng, DAI Xin. The influence of atropine on the development of myopia in children and adolescents[ J]. Journal of Heze Medical College, 2021, 33(2): 33-34.
15、国务院办公厅. 国务院办公厅关于印发国家组织药品集中采购和使用试点方案的通知[ J]. 中华人民共和国国务院公报, 2019(3): 12-15.
The General Office of the State Council. Circular of The General Office of the State Council on printing and distributing the Pilot Program for centralized Procurement and Use of Drugs organized by the State[ J]. State Council Bulletin, 2019(3): 12-15.国务院办公厅. 国务院办公厅关于印发国家组织药品集中采购和使用试点方案的通知[ J]. 中华人民共和国国务院公报, 2019(3): 12-15.
The General Office of the State Council. Circular of The General Office of the State Council on printing and distributing the Pilot Program for centralized Procurement and Use of Drugs organized by the State[ J]. State Council Bulletin, 2019(3): 12-15.