1、Suzuki M, Nagai N, Minami S, et al. Predicting recurrences of macular edema due to branch retinal vein occlusion during anti-vascular endothelial growth factor therapy[ J]. Graefes Arch Clin Exp
Ophthalmol, 2020, 258(1): 49-56.Suzuki M, Nagai N, Minami S, et al. Predicting recurrences of macular edema due to branch retinal vein occlusion during anti-vascular endothelial growth factor therapy[ J]. Graefes Arch Clin Exp
Ophthalmol, 2020, 258(1): 49-56.
2、Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion[ J]. N Engl
J Med, 2010, 363(22): 2135-2144.Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion[ J]. N Engl
J Med, 2010, 363(22): 2135-2144.
3、Lashay A, Riazi-Esfahani H, Mirghorbani M, et al. Intravitreal
medications for retinal vein occlusion: systematic review and meta�analysis[ J]. J Ophthalmic Vis Res, 2019, 14(3): 336-366.Lashay A, Riazi-Esfahani H, Mirghorbani M, et al. Intravitreal
medications for retinal vein occlusion: systematic review and meta�analysis[ J]. J Ophthalmic Vis Res, 2019, 14(3): 336-366.
4、Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, et al. Guidelines for
the management of retinal vein occlusion by the European society of
retina specialists (EURETINA)[ J]. Ophthalmologica, 2019, 242(3):
123-162.Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, et al. Guidelines for
the management of retinal vein occlusion by the European society of
retina specialists (EURETINA)[ J]. Ophthalmologica, 2019, 242(3):
123-162.
5、Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal vein occlusions
preferred practice pattern?[ J]. Ophthalmology, 2020, 127(2):
P288-P320.Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal vein occlusions
preferred practice pattern?[ J]. Ophthalmology, 2020, 127(2):
P288-P320.
6、Stewart MW. The expanding role of vascular endothelial growth factor
inhibitors in ophthalmology[ J]. Mayo Clin Proc, 2012, 87(1): 77-88.Stewart MW. The expanding role of vascular endothelial growth factor
inhibitors in ophthalmology[ J]. Mayo Clin Proc, 2012, 87(1): 77-88.
7、Berger AR, Cruess AF, Altomare F, et al. Optimal treatment of retinal
vein occlusion: canadian expert consensus[ J]. Ophthalmologica, 2015,
234(1): 6-25.Berger AR, Cruess AF, Altomare F, et al. Optimal treatment of retinal
vein occlusion: canadian expert consensus[ J]. Ophthalmologica, 2015,
234(1): 6-25.
8、Sun Z, Zhou H, Lin B, et al. Efficacy and safety of intravitreal
conbercept injections in macular edema secondary to retinal vein
occlusion[ J]. Retina, 2017, 37(9): 1723-1730.Sun Z, Zhou H, Lin B, et al. Efficacy and safety of intravitreal
conbercept injections in macular edema secondary to retinal vein
occlusion[ J]. Retina, 2017, 37(9): 1723-1730.
9、Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular
edema following central retinal vein occlusion: six-month primary end
point results of a phase III study[ J]. Ophthalmology, 2010, 117(6):
1124-1133.e1.Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular
edema following central retinal vein occlusion: six-month primary end
point results of a phase III study[ J]. Ophthalmology, 2010, 117(6):
1124-1133.e1.
10、Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular
edema following branch retinal vein occlusion: six-month primary end
point results of a phase III study[ J]. Ophthalmology, 2010, 117(6):
1102-1112.e1.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular
edema following branch retinal vein occlusion: six-month primary end
point results of a phase III study[ J]. Ophthalmology, 2010, 117(6):
1102-1112.e1.
11、Boyer D, Heier J, Brown D M, et al. Vascular endothelial growth
factor Trap-Eye for macular edema secondary to central retinal vein
occlusion: six-month results of the phase 3 COPERNICUS study[ J].
Ophthalmology, 2012, 119(5): 1024-1032.Boyer D, Heier J, Brown D M, et al. Vascular endothelial growth
factor Trap-Eye for macular edema secondary to central retinal vein
occlusion: six-month results of the phase 3 COPERNICUS study[ J].
Ophthalmology, 2012, 119(5): 1024-1032.
12、Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for
macular edema following branch retinal vein occlusion: the 24-week results
of the VIBRANT study[J]. Ophthalmology, 2015, 122(3): 538-544.Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for
macular edema following branch retinal vein occlusion: the 24-week results
of the VIBRANT study[J]. Ophthalmology, 2015, 122(3): 538-544.
13、Holz FG, Roider J, Ogura Y, et al. VEGF trap-eye for macular oedema
secondary to central retinal vein occlusion: 6-month results of the
phase III GALILEO study[ J]. Br J Ophthalmol, 2013, 97(3): 278-284.Holz FG, Roider J, Ogura Y, et al. VEGF trap-eye for macular oedema
secondary to central retinal vein occlusion: 6-month results of the
phase III GALILEO study[ J]. Br J Ophthalmol, 2013, 97(3): 278-284.
14、Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs
aflibercept on visual acuity among patients with macular edema due to
central retinal vein occlusion: the SCORE2 randomized clinical trial[ J].
JAMA, 2017, 317(20): 2072-2087.Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs
aflibercept on visual acuity among patients with macular edema due to
central retinal vein occlusion: the SCORE2 randomized clinical trial[ J].
JAMA, 2017, 317(20): 2072-2087.
15、Saishin Y, Ito Y, Fujikawa M, et al. Comparison between ranibizumab
and aflibercept for macular edema associated with central retinal vein
occlusion[ J]. Jpn J Ophthalmol, 2017, 61(1): 67-73.Saishin Y, Ito Y, Fujikawa M, et al. Comparison between ranibizumab
and aflibercept for macular edema associated with central retinal vein
occlusion[ J]. Jpn J Ophthalmol, 2017, 61(1): 67-73.
16、Thach AB, Yau L, Hoang C, et al. Time to clinically significant visual acuity
gains after ranibizumab treatment for retinal vein occlusion: BRAVO and
CRUISE trials[J]. Ophthalmology, 2014, 121(5): 1059-1066.Thach AB, Yau L, Hoang C, et al. Time to clinically significant visual acuity
gains after ranibizumab treatment for retinal vein occlusion: BRAVO and
CRUISE trials[J]. Ophthalmology, 2014, 121(5): 1059-1066.
17、Ahn SJ, Ahn J, Woo SJ, et al. Initial dose of three monthly intravitreal
injections versus PRN intravitreal injections of bevacizumab for
macular edema secondary to branch retinal vein occlusion[ J]. Biomed
Res Int, 2013, 2013: 209735.Ahn SJ, Ahn J, Woo SJ, et al. Initial dose of three monthly intravitreal
injections versus PRN intravitreal injections of bevacizumab for
macular edema secondary to branch retinal vein occlusion[ J]. Biomed
Res Int, 2013, 2013: 209735.
18、Campochiaro PA, Wykoff CC, Singer M, et al. Monthly versus as�needed ranibizumab injections in patients with retinal vein occlusion:
the SHORE study[ J]. Ophthalmology, 2014, 121(12): 2432-2442.Campochiaro PA, Wykoff CC, Singer M, et al. Monthly versus as�needed ranibizumab injections in patients with retinal vein occlusion:
the SHORE study[ J]. Ophthalmology, 2014, 121(12): 2432-2442.
19、Narayanan R, Panchal B, Das T, et al. A randomised, double-masked,
controlled study of the efficacy and safety of intravitreal bevacizumab
versus ranibizumab in the treatment of macular oedema due to branch
retinal vein occlusion: MARVEL Report No. 1[ J]. Br J Ophthalmol,
2015, 99(7): 954-959.Narayanan R, Panchal B, Das T, et al. A randomised, double-masked,
controlled study of the efficacy and safety of intravitreal bevacizumab
versus ranibizumab in the treatment of macular oedema due to branch
retinal vein occlusion: MARVEL Report No. 1[ J]. Br J Ophthalmol,
2015, 99(7): 954-959.
20、Kinge B, Stordahl PB, Forsaa V, et al. Efficacy of ranibizumab in patients
with macular edema secondary to central retinal vein occlusion: results
from the sham-controlled ROCC study[ J]. Am J Ophthalmol, 2010,
150(3): 310-314.Kinge B, Stordahl PB, Forsaa V, et al. Efficacy of ranibizumab in patients
with macular edema secondary to central retinal vein occlusion: results
from the sham-controlled ROCC study[ J]. Am J Ophthalmol, 2010,
150(3): 310-314.
21、Hykin P, Prevost AT, Vasconcelos JC, et al. Clinical effectiveness of
intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for
macular edema secondary to central retinal vein occlusion: a randomized
clinical trial[ J]. JAMA Ophthalmol, 2019, 137(11): 1256-1264.Hykin P, Prevost AT, Vasconcelos JC, et al. Clinical effectiveness of
intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for
macular edema secondary to central retinal vein occlusion: a randomized
clinical trial[ J]. JAMA Ophthalmol, 2019, 137(11): 1256-1264.
22、Kü?ük B, Sirakaya E, Karaca C. Comparison of ranibizumab versus
aflibercept in treating macular edema among patients with serous
retinal detachment secondary to branch retinal vein occlusion[ J]. Ocul
Immunol Inflamm, 2019, Epub ahead of print.Kü?ük B, Sirakaya E, Karaca C. Comparison of ranibizumab versus
aflibercept in treating macular edema among patients with serous
retinal detachment secondary to branch retinal vein occlusion[ J]. Ocul
Immunol Inflamm, 2019, Epub ahead of print.
23、Hoerauf H, Feltgen N, Weiss C, et al. Clinical efficacy and safety of
ranibizumab versus dexamethasone for central retinal vein occlusion
(COMRADE C): a European label study[ J]. Am J Ophthalmol, 2016,
169: 258-267.Hoerauf H, Feltgen N, Weiss C, et al. Clinical efficacy and safety of
ranibizumab versus dexamethasone for central retinal vein occlusion
(COMRADE C): a European label study[ J]. Am J Ophthalmol, 2016,
169: 258-267.
24、Ito Y, Saishin Y, Sawada O, et al. Comparison of single injection and
three monthly injections of intravitreal bevacizumab for macular edema
associated with branch retinal vein occlusion[ J]. Clin Ophthalmol,
2015, 9: 175-180.Ito Y, Saishin Y, Sawada O, et al. Comparison of single injection and
three monthly injections of intravitreal bevacizumab for macular edema
associated with branch retinal vein occlusion[ J]. Clin Ophthalmol,
2015, 9: 175-180.
25、蔡骐, 周跃, 黄黎黎, 等. 康柏西普治疗视网膜分支静脉阻塞继发
黄斑水肿[ J]. 国际眼科杂志, 2018, 18(5): 922-925.
CAI Q, ZHOU Y, HUANG LL, et al. Intravitreal injection of
Conbercept for macular edema due to branch retinal vein occlusion[ J].
International Eye Science, 2018, 18(5): 922-925.蔡骐, 周跃, 黄黎黎, 等. 康柏西普治疗视网膜分支静脉阻塞继发
黄斑水肿[ J]. 国际眼科杂志, 2018, 18(5): 922-925.
CAI Q, ZHOU Y, HUANG LL, et al. Intravitreal injection of
Conbercept for macular edema due to branch retinal vein occlusion[ J].
International Eye Science, 2018, 18(5): 922-925.
26、Osaka R , Muraoka Y, Miwa Y, et al. Anti-vascular endothelial
growth factor therapy for macular edema following central retinal
vein occlusion: 1 initial injection versus 3 monthly injections[ J].
Ophthalmologica, 2018, 239(1): 27-35.Osaka R , Muraoka Y, Miwa Y, et al. Anti-vascular endothelial
growth factor therapy for macular edema following central retinal
vein occlusion: 1 initial injection versus 3 monthly injections[ J].
Ophthalmologica, 2018, 239(1): 27-35.
27、富莉莉, 荣翱, 苗林, 等. 康柏西普的两种给药方案治疗视网膜静
脉阻塞黄斑水肿的短期疗效对比观察[ J]. 临床眼科杂志, 2017,
25(6): 512-516.
FU LL, RONG A, MIAO L, et al. Comparison of short-term
efficacy of two conbercept regimens for the treatment of macular
edema secondary to retinal vein occlusion[ J]. Journal of Clinical
Ophthalmology, 2017, 25(6): 512-516.富莉莉, 荣翱, 苗林, 等. 康柏西普的两种给药方案治疗视网膜静
脉阻塞黄斑水肿的短期疗效对比观察[ J]. 临床眼科杂志, 2017,
25(6): 512-516.
FU LL, RONG A, MIAO L, et al. Comparison of short-term
efficacy of two conbercept regimens for the treatment of macular
edema secondary to retinal vein occlusion[ J]. Journal of Clinical
Ophthalmology, 2017, 25(6): 512-516.
28、Miwa Y, Muraoka Y, Osaka R, et al. Ranibizumab for macular edema
after branch retinal vein occlusion: one initial injection versus three
monthly injections[ J]. Retina, 2017, 37(4): 702-709.Miwa Y, Muraoka Y, Osaka R, et al. Ranibizumab for macular edema
after branch retinal vein occlusion: one initial injection versus three
monthly injections[ J]. Retina, 2017, 37(4): 702-709.
29、赵宏锟, 吴敏. 康柏西普不同给药方案治疗视网膜分支静脉阻
塞继发黄斑水肿[ J]. 国际眼科杂志, 2019, 19(4): 567-570.
ZHAO HK, WU M. Comparison of different dosing regimens
of intravitreal conbercept in patients with macular edema secondary
to branch retinal vein occlusion[ J]. International Eye Science, 2019,
19(4): 567-570.赵宏锟, 吴敏. 康柏西普不同给药方案治疗视网膜分支静脉阻
塞继发黄斑水肿[ J]. 国际眼科杂志, 2019, 19(4): 567-570.
ZHAO HK, WU M. Comparison of different dosing regimens
of intravitreal conbercept in patients with macular edema secondary
to branch retinal vein occlusion[ J]. International Eye Science, 2019,
19(4): 567-570.
30、Pearce I, Clemens A, Brent MH, et al. Real-world outcomes with
ranibizumab in branch retinal vein occlusion: the prospective, global,
LUMINOUS study[ J]. PLoS One, 2020, 15(6): e0234739.Pearce I, Clemens A, Brent MH, et al. Real-world outcomes with
ranibizumab in branch retinal vein occlusion: the prospective, global,
LUMINOUS study[ J]. PLoS One, 2020, 15(6): e0234739.
31、McIntosh RL, Rogers SL, Lim L, et al. Natural history of central
retinal vein occlusion: an evidence-based systematic review[ J].
Ophthalmology, 2010, 117(6): 1113-1123.e15.McIntosh RL, Rogers SL, Lim L, et al. Natural history of central
retinal vein occlusion: an evidence-based systematic review[ J].
Ophthalmology, 2010, 117(6): 1113-1123.e15.
32、Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch
retinal vein occlusion: an evidence-based systematic review[ J].
Ophthalmology, 2010, 117(6): 1094-1101.e5.Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch
retinal vein occlusion: an evidence-based systematic review[ J].
Ophthalmology, 2010, 117(6): 1094-1101.e5.
33、Tadayoni R, Waldstein SM, Boscia F, et al. Individualized stabilization
criteria-driven ranibizumab versus laser in branch retinal vein
occlusion: six-month results of BRIGHTER[ J]. Ophthalmology, 2016,
123(6): 1332-1344.Tadayoni R, Waldstein SM, Boscia F, et al. Individualized stabilization
criteria-driven ranibizumab versus laser in branch retinal vein
occlusion: six-month results of BRIGHTER[ J]. Ophthalmology, 2016,
123(6): 1332-1344.
34、Tadayoni R, Waldstein S M, Boscia F, et al. Sustained benefits of
ranibizumab with or without laser in branch retinal vein occlusion:
24-month results of the BRIGHTER study[ J]. Ophthalmology, 2017,
124(12): 1778-1787.Tadayoni R, Waldstein S M, Boscia F, et al. Sustained benefits of
ranibizumab with or without laser in branch retinal vein occlusion:
24-month results of the BRIGHTER study[ J]. Ophthalmology, 2017,
124(12): 1778-1787.
35、Wei W, Weisberger A, Zhu L, et al. Efficacy and safety of ranibizumab
in asian patients with branch retinal vein occlusion: results from the
randomized BLOSSOM study[J]. Ophthalmol Retina, 2020, 4(1): 57-66.Wei W, Weisberger A, Zhu L, et al. Efficacy and safety of ranibizumab
in asian patients with branch retinal vein occlusion: results from the
randomized BLOSSOM study[J]. Ophthalmol Retina, 2020, 4(1): 57-66.
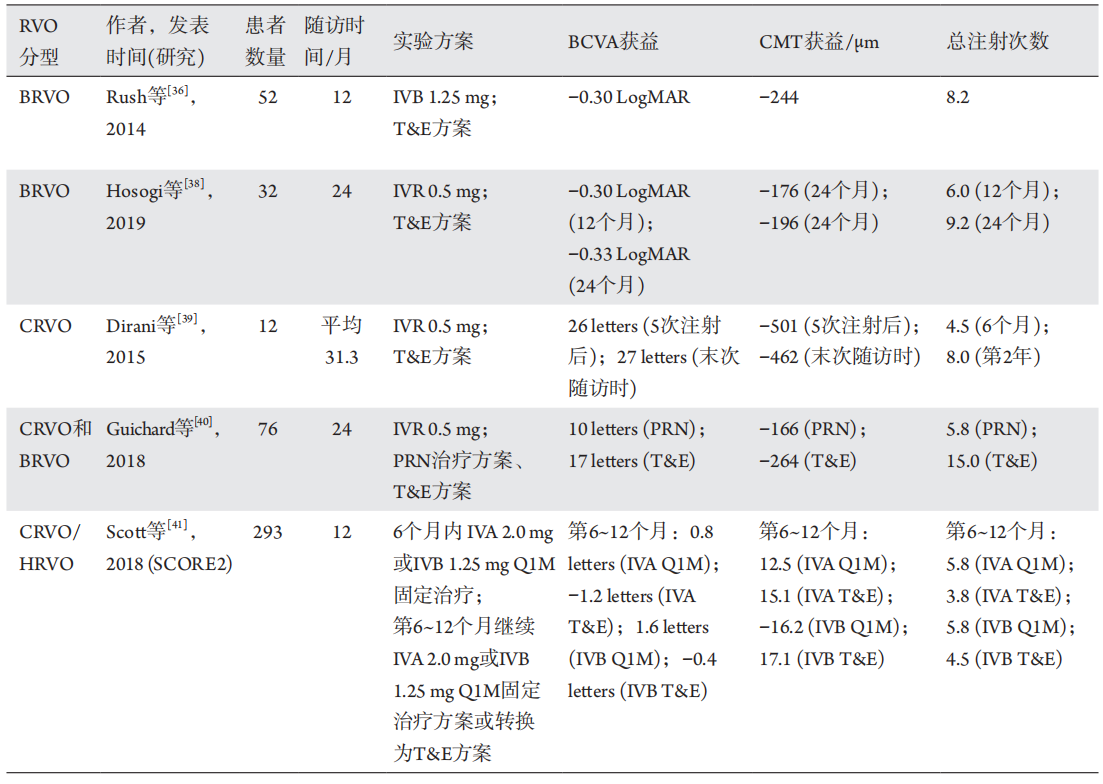
36、Rush RB, Simunovic MP, Aragon AV 2nd, et al. Treat-and-extend
intravitreal bevacizumab for branch retinal vein occlusion[ J].
Ophthalmic Surg Lasers Imaging Retina, 2014, 45(3): 212-216.Rush RB, Simunovic MP, Aragon AV 2nd, et al. Treat-and-extend
intravitreal bevacizumab for branch retinal vein occlusion[ J].
Ophthalmic Surg Lasers Imaging Retina, 2014, 45(3): 212-216.
37、Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens
with anti-VEGF agents in retinal diseases: a literature review and
consensus recommendations[ J]. Retina, 2015, 35(8): 1489-1506.Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens
with anti-VEGF agents in retinal diseases: a literature review and
consensus recommendations[ J]. Retina, 2015, 35(8): 1489-1506.
38、Hosogi M, Shiode Y, Morizane Y, et al. Two-year results of intravitreal
ranibizumab injections using a treat-and-extend regimen for macular
edema due to branch retinal vein occlusion[ J]. Acta Med Okayama,
2019, 73(6): 517-522.Hosogi M, Shiode Y, Morizane Y, et al. Two-year results of intravitreal
ranibizumab injections using a treat-and-extend regimen for macular
edema due to branch retinal vein occlusion[ J]. Acta Med Okayama,
2019, 73(6): 517-522.
39、Dirani A, Mantel I, Ambresin A. Recurrent macular edema in central
retinal vein occlusion treated with intravitreal ranibizumab using a
modified treat and extend regimen[ J]. Klin Monbl Augenheilkd, 2015,
232(4): 538-541.Dirani A, Mantel I, Ambresin A. Recurrent macular edema in central
retinal vein occlusion treated with intravitreal ranibizumab using a
modified treat and extend regimen[ J]. Klin Monbl Augenheilkd, 2015,
232(4): 538-541.
40、Guichard MM, Xavier AR, Turksever C, et al. Spectral-domain optical
coherence tomography-driven treat-and-extend and pro re nata
regimen in patients with macular oedema due to retinal vein occlusion:
24-month evaluation and outcome predictors[ J]. Ophthalmic Res,
2018, 60(1): 29-37.Guichard MM, Xavier AR, Turksever C, et al. Spectral-domain optical
coherence tomography-driven treat-and-extend and pro re nata
regimen in patients with macular oedema due to retinal vein occlusion:
24-month evaluation and outcome predictors[ J]. Ophthalmic Res,
2018, 60(1): 29-37.
41、Scott IU, VanVeldhuisen PC, Ip MS, et al. Comparison Of Monthly
Vs Treat-And-Extend Regimens For Individuals With Macular Edema
Who Respond Well To Anti-Vascular Endothelial Growth Factor
Medications: Secondary Outcomes From the SCORE2 Randomized
Clinical Trial[ J]. JAMA Ophthalmol, 2018, 136(4): 337-345.Scott IU, VanVeldhuisen PC, Ip MS, et al. Comparison Of Monthly
Vs Treat-And-Extend Regimens For Individuals With Macular Edema
Who Respond Well To Anti-Vascular Endothelial Growth Factor
Medications: Secondary Outcomes From the SCORE2 Randomized
Clinical Trial[ J]. JAMA Ophthalmol, 2018, 136(4): 337-345.
42、Wecker T, Ehlken C, Buhler A, et al. Five-year visual acuity outcomes
and injection patterns in patients with pro-re-nata treatments for
AMD, DME, RVO and myopic CNV[ J]. Br J Ophthalmol, 2017,
101(3): 353-359.Wecker T, Ehlken C, Buhler A, et al. Five-year visual acuity outcomes
and injection patterns in patients with pro-re-nata treatments for
AMD, DME, RVO and myopic CNV[ J]. Br J Ophthalmol, 2017,
101(3): 353-359.
43、Farinha C, Marques J P, Almeida E, et al. Treatment of retinal vein
occlusion with ranibizumab in clinical practice: longer-term results and
predictive factors of functional outcome[ J]. Ophthalmic Res, 2015,
55(1): 10-18.Farinha C, Marques J P, Almeida E, et al. Treatment of retinal vein
occlusion with ranibizumab in clinical practice: longer-term results and
predictive factors of functional outcome[ J]. Ophthalmic Res, 2015,
55(1): 10-18.