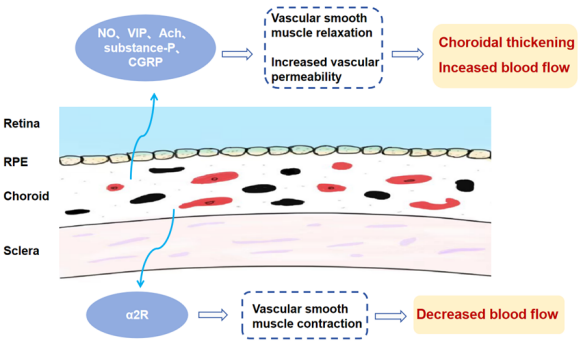
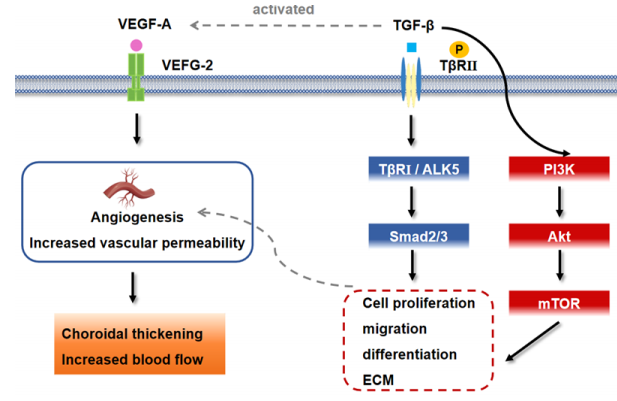
1、Li SM, Wei S, Atchison DA, et al. Annual incidences and progressions
of myopia and high myopia in Chinese schoolchildren based on a 5-year
cohort study[ J]. Invest Ophthalmol Vis Sci, 2022, 63(1): 8. DOI:
10.1167/iovs.63.1.8.Li SM, Wei S, Atchison DA, et al. Annual incidences and progressions
of myopia and high myopia in Chinese schoolchildren based on a 5-year
cohort study[ J]. Invest Ophthalmol Vis Sci, 2022, 63(1): 8. DOI:
10.1167/iovs.63.1.8.
2、Yang J, Ouyang X, Fu H, et al. Advances in biomedical study of the
myopia-related signaling pathways and mechanisms[ J]. Biomed
Pharmacother, 2022, 145: 112472. DOI: 10.1016/j.biopha.2021.112472.Yang J, Ouyang X, Fu H, et al. Advances in biomedical study of the
myopia-related signaling pathways and mechanisms[ J]. Biomed
Pharmacother, 2022, 145: 112472. DOI: 10.1016/j.biopha.2021.112472.
3、Thomson K, Kelly T, Karouta C, et al. Insights into the mechanism
by which atropine inhibits myopia: evidence against cholinergic
hyperactivity and modulation of dopamine release[ J]. Br J Pharmacol,
2021, 178(22): 4501-4517. DOI: 10.1111/bph.15629.Thomson K, Kelly T, Karouta C, et al. Insights into the mechanism
by which atropine inhibits myopia: evidence against cholinergic
hyperactivity and modulation of dopamine release[ J]. Br J Pharmacol,
2021, 178(22): 4501-4517. DOI: 10.1111/bph.15629.
4、Worthen DM. Histology of the human eye[ J]. Arch Ophthalmol, 1972,
88(2): 234. DOI: 10.1001/archopht.1972.01000030236034.Worthen DM. Histology of the human eye[ J]. Arch Ophthalmol, 1972,
88(2): 234. DOI: 10.1001/archopht.1972.01000030236034.
5、Nickla DL, Wallman J. The multifunctional choroid[ J]. Prog Retin Eye
Res, 2010, 29(2): 144-168. DOI: 10.1016/j.preteyeres.2009.12.002.Nickla DL, Wallman J. The multifunctional choroid[ J]. Prog Retin Eye
Res, 2010, 29(2): 144-168. DOI: 10.1016/j.preteyeres.2009.12.002.
6、Reiner A, Fitzgerald MEC, Del Mar N, et al. Neural control of choroidal
blood flow[ J]. Prog Retin Eye Res, 2018, 64: 96-130. DOI: 10.1016/
j.preteyeres.2017.12.001.Reiner A, Fitzgerald MEC, Del Mar N, et al. Neural control of choroidal
blood flow[ J]. Prog Retin Eye Res, 2018, 64: 96-130. DOI: 10.1016/
j.preteyeres.2017.12.001.
7、Cuthbertson S, Jackson B, Toledo C, et al. Innervation of orbital and
choroidal blood vessels by the pterygopalatine ganglion in pigeons[ J]. J
Comp Neurol, 1997, 386(3): 422-442.Cuthbertson S, Jackson B, Toledo C, et al. Innervation of orbital and
choroidal blood vessels by the pterygopalatine ganglion in pigeons[ J]. J
Comp Neurol, 1997, 386(3): 422-442.
8、Shih YF, Fitzgerald ME, Cuthbertson SL, et al. Influence of ophthalmic
nerve fibers on choroidal blood flow and myopic eye growth in chicks[ J].
Exp Eye Res, 1999, 69(1): 9-20. DOI: 10.1006/exer.1999.0692.Shih YF, Fitzgerald ME, Cuthbertson SL, et al. Influence of ophthalmic
nerve fibers on choroidal blood flow and myopic eye growth in chicks[ J].
Exp Eye Res, 1999, 69(1): 9-20. DOI: 10.1006/exer.1999.0692.
9、Schr%C3%B6dl%20F%2C%20Brehmer%20A%2C%20Neuhuber%20WL%2C%20et%20al.%20The%20autonomic%20facial%20nerve%20%0Apathway%20in%20birds%3A%20atracing%20study%20in%20chickens%5B%20J%5D.%20Invest%20Ophthalmol%20Vis%20%0ASci%2C%202006%2C%2047(8)%3A%203225-3233.%20DOI%3A%2010.1167%2Fiovs.05-1279.Schr%C3%B6dl%20F%2C%20Brehmer%20A%2C%20Neuhuber%20WL%2C%20et%20al.%20The%20autonomic%20facial%20nerve%20%0Apathway%20in%20birds%3A%20atracing%20study%20in%20chickens%5B%20J%5D.%20Invest%20Ophthalmol%20Vis%20%0ASci%2C%202006%2C%2047(8)%3A%203225-3233.%20DOI%3A%2010.1167%2Fiovs.05-1279.
10、Ramrattan RS, vander Schaft TL, Mooy CM, et al. Morphometric
analysis of Bruch's membrane, the choriocapillaris, and the choroid in
aging[ J]. InvestOphthalmolVisSci, 1994, 35(6): 2857-2864.Ramrattan RS, vander Schaft TL, Mooy CM, et al. Morphometric
analysis of Bruch's membrane, the choriocapillaris, and the choroid in
aging[ J]. InvestOphthalmolVisSci, 1994, 35(6): 2857-2864.
11、Wildsoet C, Wallman J. Choroidal and scleral mechanisms of
compensation for spectacle lenses in chicks[ J]. Vision Res, 1995, 35(9):
1175-1194. DOI: 10.1016/0042-6989(94)00233-c.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of
compensation for spectacle lenses in chicks[ J]. Vision Res, 1995, 35(9):
1175-1194. DOI: 10.1016/0042-6989(94)00233-c.
12、Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal
modulation of refractive state[ J]. Vision Res, 1995, 35(1): 37-50. DOI:
10.1016/0042-6989(94)e0049-q.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal
modulation of refractive state[ J]. Vision Res, 1995, 35(1): 37-50. DOI:
10.1016/0042-6989(94)e0049-q.
13、Tan CSH, Cheong KX. Macular choroidal thicknesses in healthy adults:
relationship with ocular and demographic factors[ J]. Invest Ophthalmol
Vis Sci, 2014, 55(10): 6452-6458. DOI: 10.1167/iovs.13-13771.Tan CSH, Cheong KX. Macular choroidal thicknesses in healthy adults:
relationship with ocular and demographic factors[ J]. Invest Ophthalmol
Vis Sci, 2014, 55(10): 6452-6458. DOI: 10.1167/iovs.13-13771.
14、Read SA, Fuss JA, Vincent SJ, et al. Choroidal changes in human myopia:
insights from optical coherence tomography imaging[ J]. Clin Exp
Optom, 2019, 102(3): 270-285. DOI: 10.1111/cxo.12862.Read SA, Fuss JA, Vincent SJ, et al. Choroidal changes in human myopia:
insights from optical coherence tomography imaging[ J]. Clin Exp
Optom, 2019, 102(3): 270-285. DOI: 10.1111/cxo.12862.
15、Wu H, Zhang G, Shen M, et al. Assessment of choroidal vascularity and
choriocapillaris blood perfusion in anisomyopic adults by SS-OCT/OCTA[ J]. Invest Ophthalmol Vis Sci, 2021, 62(1): 8. DOI: 10.1167/
iovs.62.1.8.Wu H, Zhang G, Shen M, et al. Assessment of choroidal vascularity and
choriocapillaris blood perfusion in anisomyopic adults by SS-OCT/OCTA[ J]. Invest Ophthalmol Vis Sci, 2021, 62(1): 8. DOI: 10.1167/
iovs.62.1.8.
16、Xu M, Yu X, Wan M, et al. Two-year longitudinal change in choroidal and
retinal thickness in school-aged myopic children: exploratory analysis
of clinical trials for myopia progression[ J]. Eye Vis, 2022, 9(1): 5. DOI:
10.1186/s40662-022-00276-4.Xu M, Yu X, Wan M, et al. Two-year longitudinal change in choroidal and
retinal thickness in school-aged myopic children: exploratory analysis
of clinical trials for myopia progression[ J]. Eye Vis, 2022, 9(1): 5. DOI:
10.1186/s40662-022-00276-4.
17、Fledelius HC, Jacobsen N, Li XQ, et al. Choroidal thickness at age 66
years in the Danish high myopia study cohort 1948 compared with
follow-up data on visual acuity over 40 years: a clinical update adding
spectral domain optical coherence tomography[ J]. Acta Ophthalmol,
2018, 96(1): 46-50. DOI: 10.1111/aos.13659.Fledelius HC, Jacobsen N, Li XQ, et al. Choroidal thickness at age 66
years in the Danish high myopia study cohort 1948 compared with
follow-up data on visual acuity over 40 years: a clinical update adding
spectral domain optical coherence tomography[ J]. Acta Ophthalmol,
2018, 96(1): 46-50. DOI: 10.1111/aos.13659.
18、Liu B, Wang Y, Li T, et al. Correlation of subfoveal choroidal thickness
with axial length, refractive error, and age in adult highly myopic eyes[ J].
BMC Ophthalmol, 2018, 18(1): 127. DOI: 10.1186/s12886-018-0791-5.Liu B, Wang Y, Li T, et al. Correlation of subfoveal choroidal thickness
with axial length, refractive error, and age in adult highly myopic eyes[ J].
BMC Ophthalmol, 2018, 18(1): 127. DOI: 10.1186/s12886-018-0791-5.
19、Zhou LX, Shao L, Xu L, et al. The relationship between scleral
staphyloma and choroidal thinning in highly myopic eyes: the Beijing
Eye Study[ J]. SciRep, 2017, 7(1): 9825. DOI: 10.1038/s41598-017-
10660-z.Zhou LX, Shao L, Xu L, et al. The relationship between scleral
staphyloma and choroidal thinning in highly myopic eyes: the Beijing
Eye Study[ J]. SciRep, 2017, 7(1): 9825. DOI: 10.1038/s41598-017-
10660-z.
20、Yeung SC, Park JY, Park D, et al. The effect of systemic and topical
ophthalmic medications on choroidal thickness: a review[ J].
BrJClinPharmacol, 2022, 88(6): 2673-2685. DOI: 10.1111/bcp.15237.Yeung SC, Park JY, Park D, et al. The effect of systemic and topical
ophthalmic medications on choroidal thickness: a review[ J].
BrJClinPharmacol, 2022, 88(6): 2673-2685. DOI: 10.1111/bcp.15237.
21、Zhu X, Wallman J. Opposite effects of glucagon and insulin on
compensation for spectacle lenses in chicks[ J]. InvestOphthalmolVisSci,
2009, 50(1): 24-36. DOI: 10.1167/iovs.08-1708.Zhu X, Wallman J. Opposite effects of glucagon and insulin on
compensation for spectacle lenses in chicks[ J]. InvestOphthalmolVisSci,
2009, 50(1): 24-36. DOI: 10.1167/iovs.08-1708.
22、Teberik K, Kaya M. Retinal and choroidal thickness in patients with high
myopia without maculopathy[ J]. Pak J Med Sci, 2017, 33(6): 1438-
1443. DOI: 10.12669/pjms.336.13726.Teberik K, Kaya M. Retinal and choroidal thickness in patients with high
myopia without maculopathy[ J]. Pak J Med Sci, 2017, 33(6): 1438-
1443. DOI: 10.12669/pjms.336.13726.
23、Rymer J, Wildsoet CF. The role of the retinal pigment epithelium in eye
growth regulation and myopia: a review[ J]. Vis Neurosci, 2005, 22(3):
251-261. DOI: 10.1017/S0952523805223015.Rymer J, Wildsoet CF. The role of the retinal pigment epithelium in eye
growth regulation and myopia: a review[ J]. Vis Neurosci, 2005, 22(3):
251-261. DOI: 10.1017/S0952523805223015.
24、Pendrak K, Papastergiou GI, Lin T, et al. Choroidal vascular permeability
in visually regulated eye growth[ J]. Exp Eye Res, 2000, 70(5): 629-637.
DOI: 10.1006/exer.2000.0825.Pendrak K, Papastergiou GI, Lin T, et al. Choroidal vascular permeability
in visually regulated eye growth[ J]. Exp Eye Res, 2000, 70(5): 629-637.
DOI: 10.1006/exer.2000.0825.
25、Fitzgerald MEC, Wildsoet CF, Reiner A. Temporal relationship of
choroidal blood flow and thickness changes during recovery from form
deprivation myopia in chicks[ J]. Exp Eye Res, 2002, 74(5): 561-570.
DOI: 10.1006/exer.2002.1142.Fitzgerald MEC, Wildsoet CF, Reiner A. Temporal relationship of
choroidal blood flow and thickness changes during recovery from form
deprivation myopia in chicks[ J]. Exp Eye Res, 2002, 74(5): 561-570.
DOI: 10.1006/exer.2002.1142.
26、Zhang S, Zhang G, Zhou X, et al. Changes in choroidal thickness
a n d c h o ro i d a l b l o o d p e r f u s i o n i n g u i n ea p i g my o p i a [ J] .
InvestOphthalmolVisSci, 2019, 60(8): 3074-3083. DOI: 10.1167/
iovs.18-26397.Zhang S, Zhang G, Zhou X, et al. Changes in choroidal thickness
a n d c h o ro i d a l b l o o d p e r f u s i o n i n g u i n ea p i g my o p i a [ J] .
InvestOphthalmolVisSci, 2019, 60(8): 3074-3083. DOI: 10.1167/
iovs.18-26397.
27、Cheng W, Song Y, Gao X, et al. Axial length and choriocapillaris flow
deficits in non-pathological high myopia[ J]. Am J Ophthalmol, 2022,
244: 68-78. DOI: 10.1016/j.ajo.2022.08.005.Cheng W, Song Y, Gao X, et al. Axial length and choriocapillaris flow
deficits in non-pathological high myopia[ J]. Am J Ophthalmol, 2022,
244: 68-78. DOI: 10.1016/j.ajo.2022.08.005.
28、Liu L, Zhu C, Yuan Y, et al. Three-dimensional choroidal vascularity
index in high myopia using swept-source optical coherence tomography[ J]. Curr Eye Res, 2022, 47(3): 484-492. DOI:
10.1080/02713683.2021.2006236.Liu L, Zhu C, Yuan Y, et al. Three-dimensional choroidal vascularity
index in high myopia using swept-source optical coherence tomography[ J]. Curr Eye Res, 2022, 47(3): 484-492. DOI:
10.1080/02713683.2021.2006236.
29、Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia
control[ J]. Proc Natl Acad Sci USA, 2018, 115(30): E7091-E7100. DOI:
10.1073/pnas.1721443115.Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia
control[ J]. Proc Natl Acad Sci USA, 2018, 115(30): E7091-E7100. DOI:
10.1073/pnas.1721443115.
30、Zhou X, Zhang S, Zhang G, et al. Increased choroidal blood perfusion can
inhibit form deprivation myopia in guinea pigs[ J]. Invest Ophthalmol Vis
Sci, 2020, 61(13): 25. DOI: 10.1167/iovs.61.13.25.Zhou X, Zhang S, Zhang G, et al. Increased choroidal blood perfusion can
inhibit form deprivation myopia in guinea pigs[ J]. Invest Ophthalmol Vis
Sci, 2020, 61(13): 25. DOI: 10.1167/iovs.61.13.25.
31、Zhou X, Zhang S, Yang F, et al. Decreased choroidal blood perfusion
induces myopia in guinea pigs[ J]. Invest Ophthalmol Vis Sci, 2021,
62(15): 30. DOI: 10.1167/iovs.62.15.30.Zhou X, Zhang S, Yang F, et al. Decreased choroidal blood perfusion
induces myopia in guinea pigs[ J]. Invest Ophthalmol Vis Sci, 2021,
62(15): 30. DOI: 10.1167/iovs.62.15.30.
32、Yu T, Xie X, Wei H, et al. Choroidal changes in lens-induced myopia
in guinea pigs[ J]. Microvasc Res, 2021, 138: 104213. DOI: 10.1016/
j.mvr.2021.104213.Yu T, Xie X, Wei H, et al. Choroidal changes in lens-induced myopia
in guinea pigs[ J]. Microvasc Res, 2021, 138: 104213. DOI: 10.1016/
j.mvr.2021.104213.
33、Yamamoto R, Bredt DS, Snyder SH, et al. The localization of nitric oxide
synthase in the rat eye and related cranial Ganglia[ J]. Neuroscience,
1993, 54(1): 189-200. DOI: 10.1016/0306-4522(93)90393-t.Yamamoto R, Bredt DS, Snyder SH, et al. The localization of nitric oxide
synthase in the rat eye and related cranial Ganglia[ J]. Neuroscience,
1993, 54(1): 189-200. DOI: 10.1016/0306-4522(93)90393-t.
34、Qi X, Ricart K, Ahmed KA, et al. Supplemental nitrite increases choroidal
neovascularization in mice[ J]. Nitric Oxide, 2021, 117: 7-15. DOI:
10.1016/j.niox.2021.09.005.Qi X, Ricart K, Ahmed KA, et al. Supplemental nitrite increases choroidal
neovascularization in mice[ J]. Nitric Oxide, 2021, 117: 7-15. DOI:
10.1016/j.niox.2021.09.005.
35、Erdinest N, London N, Ovadia H, et al. Nitric oxide interaction with the
eye[ J]. Vision, 2021, 5(2): 29. DOI: 10.3390/vision5020029.Erdinest N, London N, Ovadia H, et al. Nitric oxide interaction with the
eye[ J]. Vision, 2021, 5(2): 29. DOI: 10.3390/vision5020029.
36、Nickla DL, Wilken E, Lytle G, et al. Inhibiting the transient choroidal
thickening response using the nitric oxide synthase inhibitor l-NAME
prevents the ameliorative effects of visual experience on ocular growth in
two different visual paradigms[ J]. Exp Eye Res, 2006, 83(2): 456-464.
DOI: 10.1016/j.exer.2006.01.029.Nickla DL, Wilken E, Lytle G, et al. Inhibiting the transient choroidal
thickening response using the nitric oxide synthase inhibitor l-NAME
prevents the ameliorative effects of visual experience on ocular growth in
two different visual paradigms[ J]. Exp Eye Res, 2006, 83(2): 456-464.
DOI: 10.1016/j.exer.2006.01.029.
37、Nickla DL, Wildsoet CF. The effect of the nonspecific nitric oxide
synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal
compensatory response to myopic defocus in chickens[ J]. Optom Vis
Sci, 2004, 81(2): 111-118. DOI: 10.1097/00006324-200402000-00009.Nickla DL, Wildsoet CF. The effect of the nonspecific nitric oxide
synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal
compensatory response to myopic defocus in chickens[ J]. Optom Vis
Sci, 2004, 81(2): 111-118. DOI: 10.1097/00006324-200402000-00009.
38、Merkley MB, Soriano D, Jones KL, et al. The effects of nitric oxide on
choroidal gene expression[ J]. bioRxiv, 2023: 2023.06.16.545343. DOI:
10.1101/2023.06.16.545343.Merkley MB, Soriano D, Jones KL, et al. The effects of nitric oxide on
choroidal gene expression[ J]. bioRxiv, 2023: 2023.06.16.545343. DOI:
10.1101/2023.06.16.545343.
39、CarrBJ, Stell WK. Nitric oxide (NO) mediates the inhibition of form�deprivation myopia by atropine in chicks[ J]. Sci Rep, 2016, 6(1): 9.
DOI: 10.1038/s41598-016-0002-7.CarrBJ, Stell WK. Nitric oxide (NO) mediates the inhibition of form�deprivation myopia by atropine in chicks[ J]. Sci Rep, 2016, 6(1): 9.
DOI: 10.1038/s41598-016-0002-7.
40、Privalov E, Zenkel M, Schloetzer-Schrehardt U, et al. Pressure-dependent
elevation of vasoactive intestinal peptide level in chicken choroid[ J].
Biology, 2023, 12(4): 495. DOI: 10.3390/biology12040495.Privalov E, Zenkel M, Schloetzer-Schrehardt U, et al. Pressure-dependent
elevation of vasoactive intestinal peptide level in chicken choroid[ J].
Biology, 2023, 12(4): 495. DOI: 10.3390/biology12040495.
41、Nilsson SF. The significance of nitric oxide for parasympathetic
vasodilation in the eye and other orbital tissues in the cat[ J]. Exp Eye
Res, 2000, 70(1): 61-72. DOI: 10.1006/exer.1999.0752.Nilsson SF. The significance of nitric oxide for parasympathetic
vasodilation in the eye and other orbital tissues in the cat[ J]. Exp Eye
Res, 2000, 70(1): 61-72. DOI: 10.1006/exer.1999.0752.
42、Maugeri G, D'Amico AG, Saccone S, et al. PACAP and VIP inhibit
HIF-1α-mediated VEGF expression in a model of diabetic macular
edema[ J]. J Cell Physiol, 2017, 232(5): 1209-1215. DOI: 10.1002/
jcp.25616.Maugeri G, D'Amico AG, Saccone S, et al. PACAP and VIP inhibit
HIF-1α-mediated VEGF expression in a model of diabetic macular
edema[ J]. J Cell Physiol, 2017, 232(5): 1209-1215. DOI: 10.1002/
jcp.25616.
43、Liu Q, Wu J, Wang X, et al. Changes in muscarinic acetylcholine receptor
expression in form deprivation myopia in guinea pigs[ J]. Mol Vis, 2007,
13: 1234-1244.Liu Q, Wu J, Wang X, et al. Changes in muscarinic acetylcholine receptor
expression in form deprivation myopia in guinea pigs[ J]. Mol Vis, 2007,
13: 1234-1244.
44、Walch L, Brink C, Norel X. The muscarinic receptor subtypes in human
blood vessels[ J]. Therapie, 2001, 56(3): 223-226.Walch L, Brink C, Norel X. The muscarinic receptor subtypes in human
blood vessels[ J]. Therapie, 2001, 56(3): 223-226.
45、Lee SS, Lingham G, Clark A, et al. Choroidal changes during and
after discontinuing long-term 0.01% atropine treatment for myopia
control[ J]. Invest Ophthalmol Vis Sci, 2024, 65(10): 21. DOI: 10.1167/
iovs.65.10.21.Lee SS, Lingham G, Clark A, et al. Choroidal changes during and
after discontinuing long-term 0.01% atropine treatment for myopia
control[ J]. Invest Ophthalmol Vis Sci, 2024, 65(10): 21. DOI: 10.1167/
iovs.65.10.21.
46、Liu H, Chen D, Yang Z, et al. Atropine affects the outer retina during
inhibiting form deprivation myopia in guinea pigs[ J]. Curr Eye Res,
2022, 47(4): 614-623. DOI: 10.1080/02713683.2021.2009515.Liu H, Chen D, Yang Z, et al. Atropine affects the outer retina during
inhibiting form deprivation myopia in guinea pigs[ J]. Curr Eye Res,
2022, 47(4): 614-623. DOI: 10.1080/02713683.2021.2009515.
47、Stone RA, Kuwayama Y, Laties AM. Regulatory peptides in the eye[ J].
Experientia, 1987, 43(7): 791-800. DOI: 10.1007/BF01945357.Stone RA, Kuwayama Y, Laties AM. Regulatory peptides in the eye[ J].
Experientia, 1987, 43(7): 791-800. DOI: 10.1007/BF01945357.
48、May CA, Neuhuber W, Lütjen-Drecoll E. Immunohistochemical
classification and functional morphology of human choroidal ganglion
cells[ J]. Invest Ophthalmol Vis Sci, 2004, 45(2): 361-367. DOI:
10.1167/iovs.03-0624.May CA, Neuhuber W, Lütjen-Drecoll E. Immunohistochemical
classification and functional morphology of human choroidal ganglion
cells[ J]. Invest Ophthalmol Vis Sci, 2004, 45(2): 361-367. DOI:
10.1167/iovs.03-0624.
49、Schr%C3%B6dl%20F%2C%20de%20Laet%20A%2C%20Tassignon%20MJ%2C%20et%20al.%20Intrinsic%20choroidal%20neurons%20%0Ain%20the%20human%20eye%3A%20projections%2C%20targets%2C%20and%20basic%20electrophysiological%20%0Adata%5B%20J%5D.%20InvestOphthalmolVisSci%2C%202003%2C%2044(9)%3A%203705-3712.%20DOI%3A%20%0A10.1167%2Fiovs.03-0232.Schr%C3%B6dl%20F%2C%20de%20Laet%20A%2C%20Tassignon%20MJ%2C%20et%20al.%20Intrinsic%20choroidal%20neurons%20%0Ain%20the%20human%20eye%3A%20projections%2C%20targets%2C%20and%20basic%20electrophysiological%20%0Adata%5B%20J%5D.%20InvestOphthalmolVisSci%2C%202003%2C%2044(9)%3A%203705-3712.%20DOI%3A%20%0A10.1167%2Fiovs.03-0232.
50、Flügel C, Tamm ER, Mayer B, et al. Species differences in choroidal
vasodilative innervation: evidence for specific intrinsic nitrergic and VIP�positive neurons in the human eye[ J]. InvestOphthalmolVisSci, 1994,
35(2): 592-599.Flügel C, Tamm ER, Mayer B, et al. Species differences in choroidal
vasodilative innervation: evidence for specific intrinsic nitrergic and VIP�positive neurons in the human eye[ J]. InvestOphthalmolVisSci, 1994,
35(2): 592-599.
51、Schroedl F, de Stefano ME, Reese S, et al. Comparative anatomy of
nitrergic intrinsic choroidal neurons (ICN) in various avian species[ J].
Exp Eye Res, 2004, 78(2): 187-196. DOI: 10.1016/j.exer.2003.11.007.Schroedl F, de Stefano ME, Reese S, et al. Comparative anatomy of
nitrergic intrinsic choroidal neurons (ICN) in various avian species[ J].
Exp Eye Res, 2004, 78(2): 187-196. DOI: 10.1016/j.exer.2003.11.007.
52、Meriney SD, Pilar G. Cholinergic innervation of the smooth muscle
cells in the choroid coat of the chick eye and its development[ J]. J
Neurosci, 1987, 7(12): 3827-3839. DOI: 10.1523/JNEUROSCI.07- 12-
03827.1987.Meriney SD, Pilar G. Cholinergic innervation of the smooth muscle
cells in the choroid coat of the chick eye and its development[ J]. J
Neurosci, 1987, 7(12): 3827-3839. DOI: 10.1523/JNEUROSCI.07- 12-
03827.1987.
53、Poukens V, Glasgow BJ, Demer JL. Nonvascular contractile cells in sclera
and choroid of humans and monkeys[ J]. Invest Ophthalmol Vis Sci,
1998, 39(10): 1765-1774.Poukens V, Glasgow BJ, Demer JL. Nonvascular contractile cells in sclera
and choroid of humans and monkeys[ J]. Invest Ophthalmol Vis Sci,
1998, 39(10): 1765-1774.
54、Frank RN, Amin RH, Eliott D, et al. Basic fibroblast growth factor and
vascular endothelial growth factor are present in epiretinal and choroidal
neovascular membranes[ J]. Am J Ophthalmol, 1996, 122(3): 393-403.
DOI: 10.1016/s0002-9394(14)72066-5.Frank RN, Amin RH, Eliott D, et al. Basic fibroblast growth factor and
vascular endothelial growth factor are present in epiretinal and choroidal
neovascular membranes[ J]. Am J Ophthalmol, 1996, 122(3): 393-403.
DOI: 10.1016/s0002-9394(14)72066-5.
55、Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression
and receptor activation in the choroid during development and in the
adult[ J]. Invest Ophthalmol Vis Sci, 2006, 47(7): 3135-3142. DOI:
10.1167/iovs.05-1229.Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression
and receptor activation in the choroid during development and in the
adult[ J]. Invest Ophthalmol Vis Sci, 2006, 47(7): 3135-3142. DOI:
10.1167/iovs.05-1229.
56、Mathis U, Schaeffel F. Transforming growth factor-beta in the chicken
fundal layers: an immunohistochemical study[ J]. Exp Eye Res, 2010,
90(6): 780-790. DOI: 10.1016/j.exer.2010.03.014.Mathis U, Schaeffel F. Transforming growth factor-beta in the chicken
fundal layers: an immunohistochemical study[ J]. Exp Eye Res, 2010,
90(6): 780-790. DOI: 10.1016/j.exer.2010.03.014.
57、Suo NC, Lei CL, Zhang YC, et al. Effects of latanoprost on the expression
of TGF-β1 and Wnt/β-catenin signaling pathway in the choroid of
form-deprivation myopia rats[ J]. Cell Mol Biol, 2020, 66(6): 71-75.Suo NC, Lei CL, Zhang YC, et al. Effects of latanoprost on the expression
of TGF-β1 and Wnt/β-catenin signaling pathway in the choroid of
form-deprivation myopia rats[ J]. Cell Mol Biol, 2020, 66(6): 71-75.
58、Simon P, Feldkaemper M, Bitzer M, et al. Early transcriptional changes
of retinal and choroidal TGF beta-2, RALDH-2, and ZENK following
imposed positive and negative defocus in chickens[ J]. Mol Vis, 2004, 10:
588-597.Simon P, Feldkaemper M, Bitzer M, et al. Early transcriptional changes
of retinal and choroidal TGF beta-2, RALDH-2, and ZENK following
imposed positive and negative defocus in chickens[ J]. Mol Vis, 2004, 10:
588-597.
59、Li T, Bao B, Hao Y, et al. Suppressive effect of nitric oxide synthase
(NOS) inhibitor L-NMMA acetate on choroidal fibrosis in experimental
myopic guinea pigs through the nitric oxide signaling pathway[ J]. Eur J
Pharmacol, 2023, 960: 176111. DOI: 10.1016/j.ejphar.2023.176111.Li T, Bao B, Hao Y, et al. Suppressive effect of nitric oxide synthase
(NOS) inhibitor L-NMMA acetate on choroidal fibrosis in experimental
myopic guinea pigs through the nitric oxide signaling pathway[ J]. Eur J
Pharmacol, 2023, 960: 176111. DOI: 10.1016/j.ejphar.2023.176111.
60、Zhao Z, Zhang Y, Zhang C, et al. TGF-β promotes pericyte�myofibroblast transition in subretinal fibrosis through the Smad2/3 and
Akt/mTOR pathways[ J]. Exp Mol Med, 2022, 54(5): 673-684. DOI:
10.1038/s12276-022-00778-0.Zhao Z, Zhang Y, Zhang C, et al. TGF-β promotes pericyte�myofibroblast transition in subretinal fibrosis through the Smad2/3 and
Akt/mTOR pathways[ J]. Exp Mol Med, 2022, 54(5): 673-684. DOI:
10.1038/s12276-022-00778-0.
61、Yang F, Sun Y, Bai Y, et al. Asthma promotes choroidal neovascularization
via the transforming growth factor Beta1/smad signalling pathway
in a mouse model[ J]. Ophthalmic Res, 2022, 65(1): 14-29. DOI:
10.1159/000510778.Yang F, Sun Y, Bai Y, et al. Asthma promotes choroidal neovascularization
via the transforming growth factor Beta1/smad signalling pathway
in a mouse model[ J]. Ophthalmic Res, 2022, 65(1): 14-29. DOI:
10.1159/000510778.
62、Mathis U, Ziemssen F, Schaeffel F. Effects of a human VEGF antibody
(Bevacizumab) on deprivation myopia and choroidal thickness in
the chicken[ J]. Exp Eye Res, 2014, 127: 161-169. DOI: 10.1016/
j.exer.2014.07.022.Mathis U, Ziemssen F, Schaeffel F. Effects of a human VEGF antibody
(Bevacizumab) on deprivation myopia and choroidal thickness in
the chicken[ J]. Exp Eye Res, 2014, 127: 161-169. DOI: 10.1016/
j.exer.2014.07.022.
63、Uemura A, Fruttiger M, D'Amore PA, et al. VEGFR1 signaling in retinal
angiogenesis and microinflammation[ J]. Prog Retin Eye Res, 2021, 84:
100954. DOI: 10.1016/j.preteyeres.2021.100954.Uemura A, Fruttiger M, D'Amore PA, et al. VEGFR1 signaling in retinal
angiogenesis and microinflammation[ J]. Prog Retin Eye Res, 2021, 84:
100954. DOI: 10.1016/j.preteyeres.2021.100954.
64、Wang X , Ma W, Han S, et al. TGF-β par ticipates choroid
neovascularization through Smad2/3-VEGF/TNF-α signaling in mice
with Laser-induced wet age-related macular degeneration[ J]. Sci Rep,
2017, 7(1): 9672. DOI: 10.1038/s41598-017-10124-4.Wang X , Ma W, Han S, et al. TGF-β par ticipates choroid
neovascularization through Smad2/3-VEGF/TNF-α signaling in mice
with Laser-induced wet age-related macular degeneration[ J]. Sci Rep,
2017, 7(1): 9672. DOI: 10.1038/s41598-017-10124-4.
65、Steen B, Sejersen S, Berglin L, et al. Matrix metalloproteinases and
metalloproteinase inhibitors in choroidal neovascular membranes[ J].
InvestOphthalmolVisSci, 1998, 39(11): 2194-2200.Steen B, Sejersen S, Berglin L, et al. Matrix metalloproteinases and
metalloproteinase inhibitors in choroidal neovascular membranes[ J].
InvestOphthalmolVisSci, 1998, 39(11): 2194-2200.
66、Reif R, Qin J, An L, et al. Quantifying optical microangiography
images obtained from a spectral domain optical coherence tomography
system[ J]. Int J Biomed Imaging , 2012, 2012: 509783. DOI:
10.1155/2012/509783.Reif R, Qin J, An L, et al. Quantifying optical microangiography
images obtained from a spectral domain optical coherence tomography
system[ J]. Int J Biomed Imaging , 2012, 2012: 509783. DOI:
10.1155/2012/509783.
67、Rocholz R, Corvi F, Weichsel J, et al. OCT Angiography (OCTA) in
Retinal Diagnostics [M]//High Resolution Imaging in Microscopy
and Ophthalmology[ J]. High Resolution Imaging in Microscopy and
Ophthalmology: New Frontiers in Biomedical Optics, 2019: 135-160.Rocholz R, Corvi F, Weichsel J, et al. OCT Angiography (OCTA) in
Retinal Diagnostics [M]//High Resolution Imaging in Microscopy
and Ophthalmology[ J]. High Resolution Imaging in Microscopy and
Ophthalmology: New Frontiers in Biomedical Optics, 2019: 135-160.
68、Ostrin L A, Harb E, Nickla D L, et al. IMI: the dynamic choroid: new
insights, challenges, and potential significance for human myopia [ J].
Invest Ophthalmol Vis Sci, 2023, 64(6): 4. DOI: 10.1167/iovs.64.6.4.Ostrin L A, Harb E, Nickla D L, et al. IMI: the dynamic choroid: new
insights, challenges, and potential significance for human myopia [ J].
Invest Ophthalmol Vis Sci, 2023, 64(6): 4. DOI: 10.1167/iovs.64.6.4.