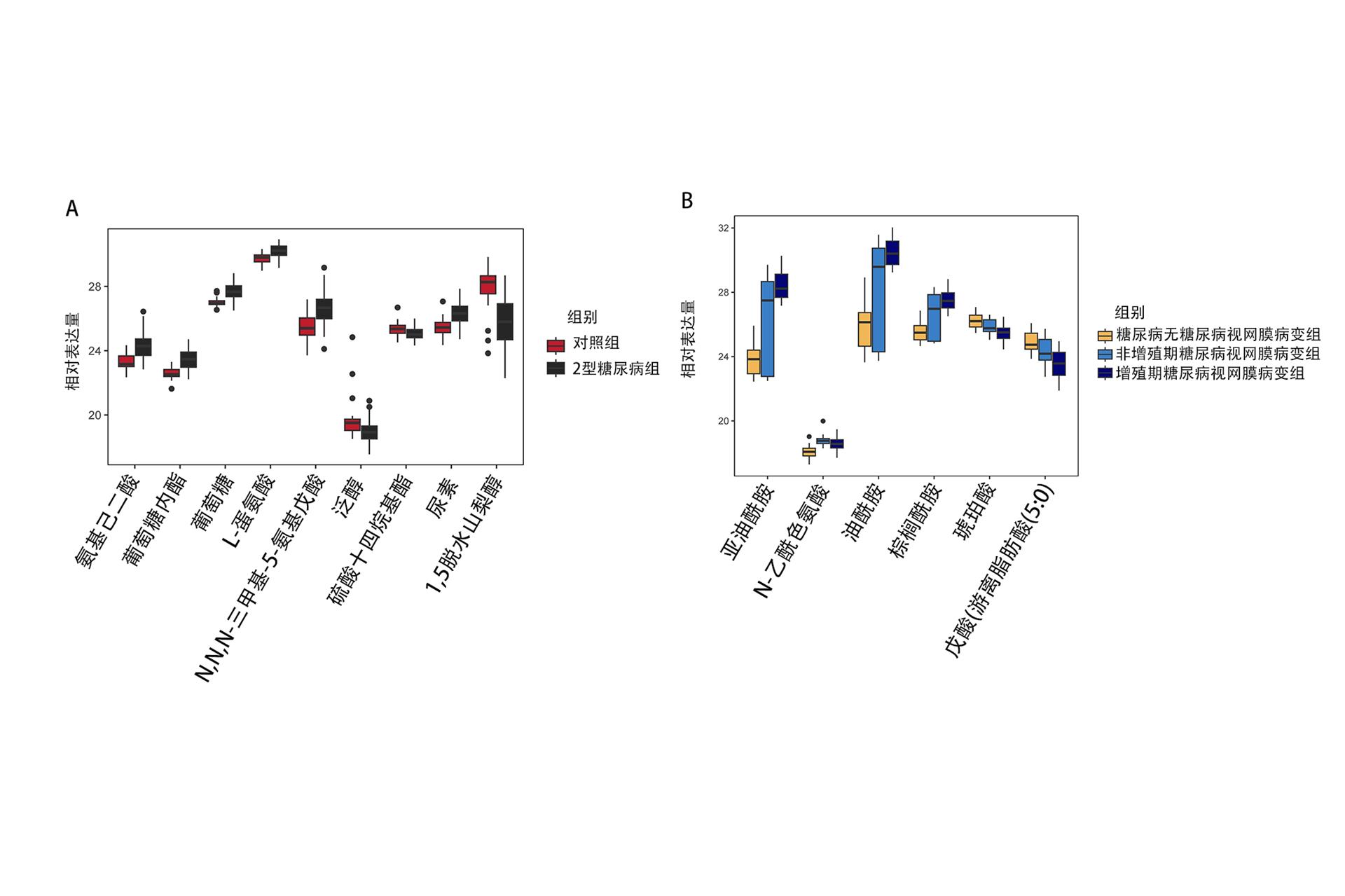
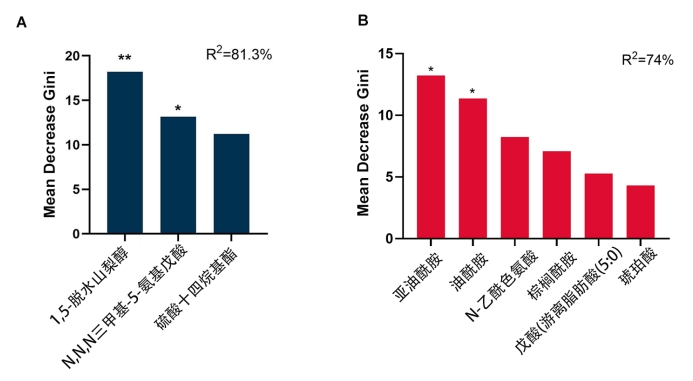
1、Risk Factor Collaboration (NCD-RisC) NCD. Worldwide trends
in diabetes prevalence and treatment from 1990 to 2022: a pooled
analysis of 1108 population-representative studies with 141 million
participants. Lancet, 2024, 404(10467): 2077-2093. DOI: 10.1016/
S0140-6736(24)02317-1.Risk Factor Collaboration (NCD-RisC) NCD. Worldwide trends
in diabetes prevalence and treatment from 1990 to 2022: a pooled
analysis of 1108 population-representative studies with 141 million
participants. Lancet, 2024, 404(10467): 2077-2093. DOI: 10.1016/
S0140-6736(24)02317-1.
2、Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet, 2010,
376(9735): 124-136. DOI: 10.1016/S0140-6736(09)62124-3.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet, 2010,
376(9735): 124-136. DOI: 10.1016/S0140-6736(09)62124-3.
3、Hou X, Wang L, Zhu D, et al. Prevalence of diabetic retinopathy and vision�threatening diabetic retinopathy in adults with diabetes in China. Nat
Commun, 2023, 14(1): 4296. DOI: 10.1038/s41467-023-39864-w.Hou X, Wang L, Zhu D, et al. Prevalence of diabetic retinopathy and vision�threatening diabetic retinopathy in adults with diabetes in China. Nat
Commun, 2023, 14(1): 4296. DOI: 10.1038/s41467-023-39864-w.
4、Chai YH, Zhang YP, Qiao YS, et al. Association between diabetic
retinopathy, brain structural abnormalities, and cognitive impairment for accumulated evidence in observational studies. Am J Ophthalmol,
2022, 239: 37-53. DOI: 10.1016/j.ajo.2022.01.011.Chai YH, Zhang YP, Qiao YS, et al. Association between diabetic
retinopathy, brain structural abnormalities, and cognitive impairment for accumulated evidence in observational studies. Am J Ophthalmol,
2022, 239: 37-53. DOI: 10.1016/j.ajo.2022.01.011.
5、Kim HU, Park SP, Kim YK. Long-term HbA1c variability and the
development and progression of diabetic retinopathy in subjects with
type 2 diabetes. Sci Rep, 2021, 11(1): 4731. DOI: 10.1038/s41598-
021-84150-8.Kim HU, Park SP, Kim YK. Long-term HbA1c variability and the
development and progression of diabetic retinopathy in subjects with
type 2 diabetes. Sci Rep, 2021, 11(1): 4731. DOI: 10.1038/s41598-
021-84150-8.
6、Gonz%C3%A1lez-Pe%C3%B1a%20D%2C%20Brennan%20L.%20Recent%20advances%20in%20the%20application%20of%20%0Ametabolomics%20for%20nutrition%20and%20health.%20Annu%20Rev%20Food%20Sci%20Technol%2C%20%0A2019%2C%2010%3A%20479-519.%20DOI%3A%2010.1146%2Fannurev-food-032818-121715.Gonz%C3%A1lez-Pe%C3%B1a%20D%2C%20Brennan%20L.%20Recent%20advances%20in%20the%20application%20of%20%0Ametabolomics%20for%20nutrition%20and%20health.%20Annu%20Rev%20Food%20Sci%20Technol%2C%20%0A2019%2C%2010%3A%20479-519.%20DOI%3A%2010.1146%2Fannurev-food-032818-121715.
7、Zhang J, Zhang J, Zhang C, et al. Diabetic macular edema: current
understanding, molecular mechanisms and therapeutic implications.
Cells, 2022, 11(21): 3362. DOI: 10.3390/cells11213362.Zhang J, Zhang J, Zhang C, et al. Diabetic macular edema: current
understanding, molecular mechanisms and therapeutic implications.
Cells, 2022, 11(21): 3362. DOI: 10.3390/cells11213362.
8、Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond
biomarkers and towards mechanisms. Nat Rev Mol Cell Biol, 2016,
17(7): 451-459. DOI: 10.1038/nrm.2016.25.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond
biomarkers and towards mechanisms. Nat Rev Mol Cell Biol, 2016,
17(7): 451-459. DOI: 10.1038/nrm.2016.25.
9、Peters KS, Rivera E, Warden C, et al. Plasma arginine and citrulline are
elevated in diabetic retinopathy. Am J Ophthalmol, 2022, 235: 154-162.
DOI: 10.1016/j.ajo.2021.09.021.Peters KS, Rivera E, Warden C, et al. Plasma arginine and citrulline are
elevated in diabetic retinopathy. Am J Ophthalmol, 2022, 235: 154-162.
DOI: 10.1016/j.ajo.2021.09.021.
10、Sumarriva K, Uppal K, Ma C, et al. Arginine and carnitine metabolites
are altered in diabetic retinopathy. Invest Ophthalmol Vis Sci, 2019,
60(8): 3119-3126. DOI: 10.1167/iovs.19-27321.Sumarriva K, Uppal K, Ma C, et al. Arginine and carnitine metabolites
are altered in diabetic retinopathy. Invest Ophthalmol Vis Sci, 2019,
60(8): 3119-3126. DOI: 10.1167/iovs.19-27321.
11、Rhee SY, Jung ES, Park HM, et al. Plasma glutamine and glutamic
acid are potential biomarkers for predicting diabetic retinopathy.
Metabolomics, 2018, 14(7): 89. DOI: 10.1007/s11306-018-1383-3.Rhee SY, Jung ES, Park HM, et al. Plasma glutamine and glutamic
acid are potential biomarkers for predicting diabetic retinopathy.
Metabolomics, 2018, 14(7): 89. DOI: 10.1007/s11306-018-1383-3.
12、Sun Y, Zou H, Li X, et al. Plasma metabolomics reveals metabolic
profiling for diabetic retinopathy and disease progression. Front
Endocrinol (Lausanne), 2021, 12: 757088. DOI: 10.3389/
fendo.2021.757088.Sun Y, Zou H, Li X, et al. Plasma metabolomics reveals metabolic
profiling for diabetic retinopathy and disease progression. Front
Endocrinol (Lausanne), 2021, 12: 757088. DOI: 10.3389/
fendo.2021.757088.
13、Wang Z, Tang J, Jin E, et al. Serum untargeted metabolomics reveal
potential biomarkers of progression of diabetic retinopathy in asians.
Front Mol Biosci, 2022, 9: 871291. DOI: 10.3389/fmolb.2022.871291.Wang Z, Tang J, Jin E, et al. Serum untargeted metabolomics reveal
potential biomarkers of progression of diabetic retinopathy in asians.
Front Mol Biosci, 2022, 9: 871291. DOI: 10.3389/fmolb.2022.871291.
14、Wang X, Yang S, Yang G, et al. Novel risk score model for non�proliferative diabetic retinopathy based on untargeted metabolomics of
venous blood. Front Endocrinol (Lausanne), 2023, 14: 1180415. DOI:
10.3389/fendo.2023.1180415.Wang X, Yang S, Yang G, et al. Novel risk score model for non�proliferative diabetic retinopathy based on untargeted metabolomics of
venous blood. Front Endocrinol (Lausanne), 2023, 14: 1180415. DOI:
10.3389/fendo.2023.1180415.
15、Zhu XR , Yang FY, Lu J, et al. Plasma metabolomic profiling of
proliferative diabetic retinopathy. Nutr Metab (Lond), 2019, 16: 37.
DOI: 10.1186/s12986-019-0358-3.Zhu XR , Yang FY, Lu J, et al. Plasma metabolomic profiling of
proliferative diabetic retinopathy. Nutr Metab (Lond), 2019, 16: 37.
DOI: 10.1186/s12986-019-0358-3.
16、Guo C, Jiang D, Xu Y, et al. High-coverage serum metabolomics reveals
metabolic pathway dysregulation in diabetic retinopathy: a propensity
score-matched study. Front Mol Biosci, 2022, 9: 822647. DOI:
10.3389/fmolb.2022.822647.Guo C, Jiang D, Xu Y, et al. High-coverage serum metabolomics reveals
metabolic pathway dysregulation in diabetic retinopathy: a propensity
score-matched study. Front Mol Biosci, 2022, 9: 822647. DOI:
10.3389/fmolb.2022.822647.
17、Zuo J, Lan Y, Hu H, et al. Metabolomics-based multidimensional
network biomarkers for diabetic retinopathy identification in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care, 2021,
9(1): e001443. DOI: 10.1136/bmjdrc-2020-001443.Zuo J, Lan Y, Hu H, et al. Metabolomics-based multidimensional
network biomarkers for diabetic retinopathy identification in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care, 2021,
9(1): e001443. DOI: 10.1136/bmjdrc-2020-001443.
18、Han X, Zhang L, Kong L, et al. Comprehensive metabolic profiling of
diabetic retinopathy. Exp Eye Res, 2023, 233: 109538. DOI: 10.1016/
j.exer.2023.109538.Han X, Zhang L, Kong L, et al. Comprehensive metabolic profiling of
diabetic retinopathy. Exp Eye Res, 2023, 233: 109538. DOI: 10.1016/
j.exer.2023.109538.
19、Liu H, Li J, Wong L. A comparative study on feature selection and
classification methods using gene expression profiles and proteomic
patterns. Genome Inform, 2002, 13: 51-60.Liu H, Li J, Wong L. A comparative study on feature selection and
classification methods using gene expression profiles and proteomic
patterns. Genome Inform, 2002, 13: 51-60.
20、Strobl C, Boulesteix AL, Kneib T, et al. Conditional variable
importance for random forests. BMC Bioinformatics, 2008, 9: 307.
DOI: 10.1186/1471-2105-9-307.Strobl C, Boulesteix AL, Kneib T, et al. Conditional variable
importance for random forests. BMC Bioinformatics, 2008, 9: 307.
DOI: 10.1186/1471-2105-9-307.
21、Sammut SJ, Crispin-Ortuzar M, Chin SF, et al. Multi-omic machine
learning predictor of breast cancer therapy response. Nature, 2022,
601(7894): 623-629. DOI: 10.1038/s41586-021-04278-5.Sammut SJ, Crispin-Ortuzar M, Chin SF, et al. Multi-omic machine
learning predictor of breast cancer therapy response. Nature, 2022,
601(7894): 623-629. DOI: 10.1038/s41586-021-04278-5.
22、Lewis JE, Kemp ML. Integration of machine learning and genome-scale
metabolic modeling identifies multi-omics biomarkers for radiation
resistance. Nat Commun, 2021, 12(1): 2700. DOI: 10.1038/s41467-
021-22989-1.Lewis JE, Kemp ML. Integration of machine learning and genome-scale
metabolic modeling identifies multi-omics biomarkers for radiation
resistance. Nat Commun, 2021, 12(1): 2700. DOI: 10.1038/s41467-
021-22989-1.
23、Yamanouchi T, Ogata N, Tagaya T, et al. Clinical usefulness of serum
1, 5-anhydroglucitol in monitoring glycaemic control. Lancet, 1996,
347(9014): 1514-1518. DOI: 10.1016/s0140-6736(96)90672-8.Yamanouchi T, Ogata N, Tagaya T, et al. Clinical usefulness of serum
1, 5-anhydroglucitol in monitoring glycaemic control. Lancet, 1996,
347(9014): 1514-1518. DOI: 10.1016/s0140-6736(96)90672-8.
24、Dungan KM. 1, 5-anhydroglucitol (GlycoMark) as a marker of short�term glycemic control and glycemic excursions. Expert Rev Mol Diagn,
2008, 8(1): 9-19. DOI: 10.1586/14737159.8.1.9.Dungan KM. 1, 5-anhydroglucitol (GlycoMark) as a marker of short�term glycemic control and glycemic excursions. Expert Rev Mol Diagn,
2008, 8(1): 9-19. DOI: 10.1586/14737159.8.1.9.
25、Selv in E, R aw lings A M, Grams M, et al. A ssoc iation of 1,
5-anhydroglucitol with diabetes and microvascular conditions.
Clin Chem, 2014, 60(11): 1409-1418. DOI: 10.1373/
clinchem.2014.229427.Selv in E, R aw lings A M, Grams M, et al. A ssoc iation of 1,
5-anhydroglucitol with diabetes and microvascular conditions.
Clin Chem, 2014, 60(11): 1409-1418. DOI: 10.1373/
clinchem.2014.229427.
26、Shen Y, Si Y, Lu J, et al. Association between 1, 5-anhydroglucitol
and acute C peptide response to arginine among patients with
type 2 diabetes. J Diabetes Res, 2020, 2020: 4243053. DOI:
10.1155/2020/4243053.Shen Y, Si Y, Lu J, et al. Association between 1, 5-anhydroglucitol
and acute C peptide response to arginine among patients with
type 2 diabetes. J Diabetes Res, 2020, 2020: 4243053. DOI:
10.1155/2020/4243053.
27、Zhao M, Wei H, Li C, et al. Gut microbiota production of trimethyl-5-
aminovaleric acid reduces fatty acid oxidation and accelerates cardiac
hypertrophy. Nat Commun, 2022, 13(1): 1757. DOI: 10.1038/s41467-
022-29060-7.Zhao M, Wei H, Li C, et al. Gut microbiota production of trimethyl-5-
aminovaleric acid reduces fatty acid oxidation and accelerates cardiac
hypertrophy. Nat Commun, 2022, 13(1): 1757. DOI: 10.1038/s41467-
022-29060-7.
28、Yamamoto S, Takehara M, Ushimaru M. Inhibitor y action of
linoleamide and oleamide toward sarco/endoplasmic reticulum Ca2+-
ATPase. Biochim Biophys Acta Gen Subj, 2017, 1861(1 Pt A): 3399-
3405. DOI: 10.1016/j.bbagen.2016.09.001.Yamamoto S, Takehara M, Ushimaru M. Inhibitor y action of
linoleamide and oleamide toward sarco/endoplasmic reticulum Ca2+-
ATPase. Biochim Biophys Acta Gen Subj, 2017, 1861(1 Pt A): 3399-
3405. DOI: 10.1016/j.bbagen.2016.09.001.
29、Huang JK, Jan CR. Linoleamide, a brain lipid that induces sleep,
increases cytosolic Ca2+ levels in MDCK renal tubular cells. Life Sci,
2001, 68(9): 997-1004. DOI: 10.1016/s0024-3205(00)01002-x.Huang JK, Jan CR. Linoleamide, a brain lipid that induces sleep,
increases cytosolic Ca2+ levels in MDCK renal tubular cells. Life Sci,
2001, 68(9): 997-1004. DOI: 10.1016/s0024-3205(00)01002-x.
30、Yamamoto S, Takehara M, Kabashima Y, et al. Identification of novel inhibitors of human SPCA2[ J]. Biochem Biophys Res Commun, 2016,
477(2): 266-270. DOI: 10.1016/j.bbrc.2016.06.055.Yamamoto S, Takehara M, Kabashima Y, et al. Identification of novel inhibitors of human SPCA2[ J]. Biochem Biophys Res Commun, 2016,
477(2): 266-270. DOI: 10.1016/j.bbrc.2016.06.055.
31、Yu H, Zhou D, Wang W, et al. Protective effect of baicalin on oxidative
stress injury in retinal ganglion cells through the JAK/STAT signaling
pathway in vitro and in vivo. Front Pharmacol, 2024, 15: 1443472.
DOI: 10.3389/fphar.2024.1443472.Yu H, Zhou D, Wang W, et al. Protective effect of baicalin on oxidative
stress injury in retinal ganglion cells through the JAK/STAT signaling
pathway in vitro and in vivo. Front Pharmacol, 2024, 15: 1443472.
DOI: 10.3389/fphar.2024.1443472.
32、Ibrahim WW, Sayed RH, Abdelhameed MF, et al. Neuroprotective
potential of Erigeron bonariensis ethanolic ex tract against
ovariectomized/D-galactose-induced memory impairments in female
rats in relation to its metabolite fingerprint as revealed using UPLC/
MS. Inflammopharmacology, 2024, 32(2): 1091-1112. DOI: 10.1007/
s10787-023-01418-3.Ibrahim WW, Sayed RH, Abdelhameed MF, et al. Neuroprotective
potential of Erigeron bonariensis ethanolic ex tract against
ovariectomized/D-galactose-induced memory impairments in female
rats in relation to its metabolite fingerprint as revealed using UPLC/
MS. Inflammopharmacology, 2024, 32(2): 1091-1112. DOI: 10.1007/
s10787-023-01418-3.
33、Leggett JD, Aspley S, Beckett SG, et al. Oleamide is a selective
endogenous agonist of rat and human CB1 cannabinoid receptors. Br J
Pharmacol, 2004, 141(2): 253-262. DOI: 10.1038/sj.bjp.0705607.Leggett JD, Aspley S, Beckett SG, et al. Oleamide is a selective
endogenous agonist of rat and human CB1 cannabinoid receptors. Br J
Pharmacol, 2004, 141(2): 253-262. DOI: 10.1038/sj.bjp.0705607.
34、Pawar HD, Patil Y, Patil A, et al. Cardioprotective effect of CB1 receptor
antagonist AM251 against β receptor-stimulated myocardial infarction
via modulation of NF-kB signaling pathway in diabetic mice. Heliyon,
2024, 10(15): e35138. DOI: 10.1016/j.heliyon.2024.e35138.Pawar HD, Patil Y, Patil A, et al. Cardioprotective effect of CB1 receptor
antagonist AM251 against β receptor-stimulated myocardial infarction
via modulation of NF-kB signaling pathway in diabetic mice. Heliyon,
2024, 10(15): e35138. DOI: 10.1016/j.heliyon.2024.e35138.
35、Kim M, Song G, Kang M, et al. Replacing carbohydrate with protein
and fat in prediabetes or type-2 diabetes: greater effect on metabolites in PBMC than plasma. Nutr Metab (Lond), 2016, 13: 3. DOI:
10.1186/s12986-016-0063-4.Kim M, Song G, Kang M, et al. Replacing carbohydrate with protein
and fat in prediabetes or type-2 diabetes: greater effect on metabolites in PBMC than plasma. Nutr Metab (Lond), 2016, 13: 3. DOI:
10.1186/s12986-016-0063-4.
36、Mahali SK, Manna SK. Beta-D-glucoside protects against advanced
glycation end products (AGEs)-mediated diabetic responses
by suppressing ERK and inducing PPAR gamma DNA binding.
Biochem Pharmacol, 2012, 84(12): 1681-1690. DOI: 10.1016/
j.bcp.2012.09.033.Mahali SK, Manna SK. Beta-D-glucoside protects against advanced
glycation end products (AGEs)-mediated diabetic responses
by suppressing ERK and inducing PPAR gamma DNA binding.
Biochem Pharmacol, 2012, 84(12): 1681-1690. DOI: 10.1016/
j.bcp.2012.09.033.
37、Ding X, Qiu Y, Wu G, et al. L-thyroxine attenuates extracellular
Hsp90α-induced vascular endothelial calcification in diabetes
mellitus, as revealed by parallel metabolic profiles. Atherosclerosis,
2024, 392: 117527. DOI: 10.1016/j.atherosclerosis.2024.117527.Ding X, Qiu Y, Wu G, et al. L-thyroxine attenuates extracellular
Hsp90α-induced vascular endothelial calcification in diabetes
mellitus, as revealed by parallel metabolic profiles. Atherosclerosis,
2024, 392: 117527. DOI: 10.1016/j.atherosclerosis.2024.117527.
38、Lim SK, Park MJ, Lim JC, et al. Hyperglycemia induces apoptosis via
CB1 activation through the decrease of FAAH 1 in retinal pigment
epithelial cell. J Cell Physiol, 2012, 227(2): 569-577. DOI: 10.1002/
jcp.22756.Lim SK, Park MJ, Lim JC, et al. Hyperglycemia induces apoptosis via
CB1 activation through the decrease of FAAH 1 in retinal pigment
epithelial cell. J Cell Physiol, 2012, 227(2): 569-577. DOI: 10.1002/
jcp.22756.
39、Kumar P, Kumar A, Song ZH. Structure-activity relationships of fatty
acid amide ligands in activating and desensitizing G protein-coupled
receptor 119. Eur J Pharmacol, 2014, 723: 465-472. DOI: 10.1016/
j.ejphar.2013.10.044.Kumar P, Kumar A, Song ZH. Structure-activity relationships of fatty
acid amide ligands in activating and desensitizing G protein-coupled
receptor 119. Eur J Pharmacol, 2014, 723: 465-472. DOI: 10.1016/
j.ejphar.2013.10.044.