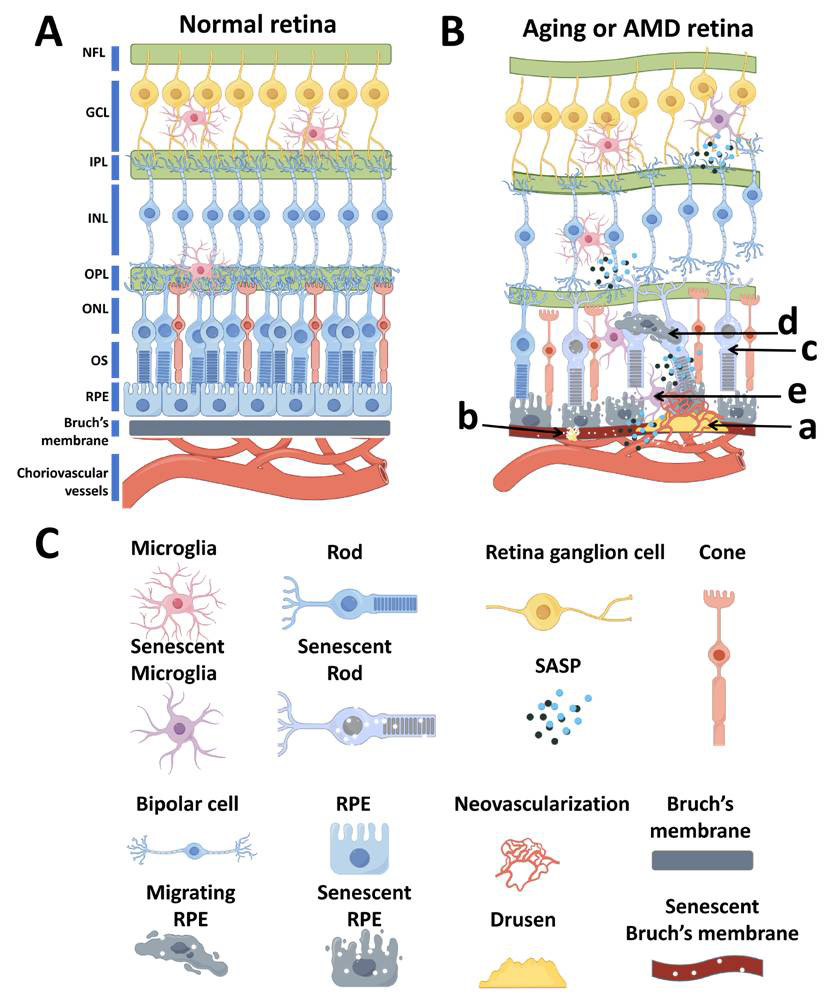
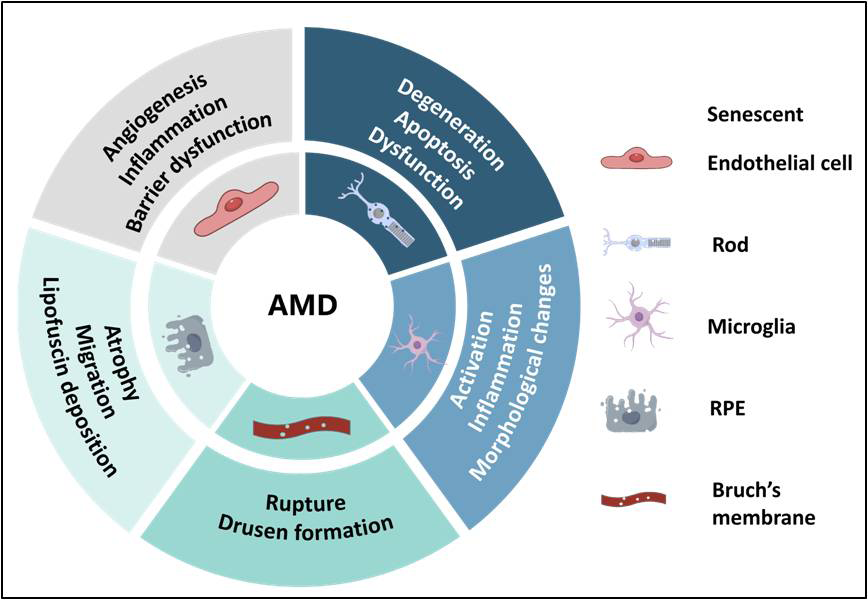
1、Guymer RH, Campbell TG. Age-related macular degeneration[ J].
Lancet, 2023, 401(10386): 1459-1472. DOI: 10.1016/s0140-
6736(22)02609-5.Guymer RH, Campbell TG. Age-related macular degeneration[ J].
Lancet, 2023, 401(10386): 1459-1472. DOI: 10.1016/s0140-
6736(22)02609-5.
2、Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-related
macular degeneration: a review[ J]. JAMA, 2024, 331(2): 147-157.
DOI: 10.1001/jama.2023.26074.Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-related
macular degeneration: a review[ J]. JAMA, 2024, 331(2): 147-157.
DOI: 10.1001/jama.2023.26074.
3、Seddon JM. Macular degeneration epidemiology: nature-nurture,
lifestyle factors, genetic risk, and gene-environment interactions–the
weisenfeld award lecture[ J]. Invest Ophthalmol Vis Sci, 2017, 58(14):6513. DOI: 10.1167/iovs.17-23544.Seddon JM. Macular degeneration epidemiology: nature-nurture,
lifestyle factors, genetic risk, and gene-environment interactions–the
weisenfeld award lecture[ J]. Invest Ophthalmol Vis Sci, 2017, 58(14):6513. DOI: 10.1167/iovs.17-23544.
4、Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and
age-related disease: from mechanisms to therapy[ J]. Nat Med, 2015,
21(12): 1424-1435. DOI: 10.1038/nm.4000.Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and
age-related disease: from mechanisms to therapy[ J]. Nat Med, 2015,
21(12): 1424-1435. DOI: 10.1038/nm.4000.
5、Gorgoulis V, Adams PD, Alimonti A, et al. Cellular senescence: defining
a path forward[ J]. Cell, 2019, 179(4): 813-827. DOI: 10.1016/
j.cell.2019.10.005.Gorgoulis V, Adams PD, Alimonti A, et al. Cellular senescence: defining
a path forward[ J]. Cell, 2019, 179(4): 813-827. DOI: 10.1016/
j.cell.2019.10.005.
6、Campisi J. Aging, cellular senescence, and cancer[ J]. Annu Rev Physiol,
2013, 75: 685-705. DOI: 10.1146/annurev-physiol-030212-183653.Campisi J. Aging, cellular senescence, and cancer[ J]. Annu Rev Physiol,
2013, 75: 685-705. DOI: 10.1146/annurev-physiol-030212-183653.
7、Malek%20G%2C%20Campisi%20J%2C%20Kitazawa%20K%2C%20et%20al.%20Does%20senescence%20play%20a%20role%20in%20%0Aage-related%20macular%20degeneration%3F%5B%20J%5D.%20Exp%20Eye%20Res%2C%202022%2C%20225%3A%20109254.%20%0ADOI%3A%2010.1016%2Fj.exer.2022.109254.Malek%20G%2C%20Campisi%20J%2C%20Kitazawa%20K%2C%20et%20al.%20Does%20senescence%20play%20a%20role%20in%20%0Aage-related%20macular%20degeneration%3F%5B%20J%5D.%20Exp%20Eye%20Res%2C%202022%2C%20225%3A%20109254.%20%0ADOI%3A%2010.1016%2Fj.exer.2022.109254.
8、Terao R, Ahmed T, Suzumura A, et al. Oxidative stress-induced cellular
senescence in aging retina and age-related macular degeneration[ J].
Antioxidants, 2022, 11(11): 2189. DOI: 10.3390/antiox11112189.Terao R, Ahmed T, Suzumura A, et al. Oxidative stress-induced cellular
senescence in aging retina and age-related macular degeneration[ J].
Antioxidants, 2022, 11(11): 2189. DOI: 10.3390/antiox11112189.
9、Blasiak J. Senescence in the pathogenesis of age-related macular
degeneration[ J]. Cell Mol Life Sci, 2020, 77(5): 789-805. DOI:
10.1007/s00018-019-03420-x.Blasiak J. Senescence in the pathogenesis of age-related macular
degeneration[ J]. Cell Mol Life Sci, 2020, 77(5): 789-805. DOI:
10.1007/s00018-019-03420-x.
10、Lee KS, Lin S, Copland DA, et al. Cellular senescence in the aging
retina and developments of senotherapies for age-related macular
degeneration[ J]. J Neuroinflammation, 2021, 18(1): 32. DOI:
10.1186/s12974-021-02088-0.Lee KS, Lin S, Copland DA, et al. Cellular senescence in the aging
retina and developments of senotherapies for age-related macular
degeneration[ J]. J Neuroinflammation, 2021, 18(1): 32. DOI:
10.1186/s12974-021-02088-0.
11、Crespo-Garcia S, Tsuruda PR, Dejda A, et al. Pathological angiogenesis
in retinopathy engages cellular senescence and is amenable to
therapeutic elimination via BCL-xL inhibition[ J]. Cell Metab, 2021,
33(4): 818-832.e7. DOI: 10.1016/j.cmet.2021.01.011.Crespo-Garcia S, Tsuruda PR, Dejda A, et al. Pathological angiogenesis
in retinopathy engages cellular senescence and is amenable to
therapeutic elimination via BCL-xL inhibition[ J]. Cell Metab, 2021,
33(4): 818-832.e7. DOI: 10.1016/j.cmet.2021.01.011.
12、Kr ytkowska E, Olejnik-Wojciechowska J, Grabowicz A , et al.
Association between subretinal drusenoid deposits and age-related
macular degeneration in multimodal retinal imaging[ J]. J Clin Med,
2023, 12(24): 7728. DOI: 10.3390/jcm12247728.Kr ytkowska E, Olejnik-Wojciechowska J, Grabowicz A , et al.
Association between subretinal drusenoid deposits and age-related
macular degeneration in multimodal retinal imaging[ J]. J Clin Med,
2023, 12(24): 7728. DOI: 10.3390/jcm12247728.
13、Zarubina AV, Neely DC, Clark ME, et al. Prevalence of subretinal
drusenoid depositsin older persons with and without age-related
macular degeneration, by multimodal imaging[ J]. Ophthalmology,
2016, 123(5): 1090-1100. DOI: 10.1016/j.ophtha.2015.12.034.Zarubina AV, Neely DC, Clark ME, et al. Prevalence of subretinal
drusenoid depositsin older persons with and without age-related
macular degeneration, by multimodal imaging[ J]. Ophthalmology,
2016, 123(5): 1090-1100. DOI: 10.1016/j.ophtha.2015.12.034.
14、Flores-Bellver M, Mighty J, Aparicio-Domingo S, et al. Extracellular
vesicles released by human retinal pigment epithelium mediate
increased polarised secretion of drusen proteins in response to AMD
stressors[ J]. J Extracell Vesicles, 2021, 10(13): e12165. DOI: 10.1002/
jev2.12165.Flores-Bellver M, Mighty J, Aparicio-Domingo S, et al. Extracellular
vesicles released by human retinal pigment epithelium mediate
increased polarised secretion of drusen proteins in response to AMD
stressors[ J]. J Extracell Vesicles, 2021, 10(13): e12165. DOI: 10.1002/
jev2.12165.
15、Khan KN, Mahroo OA, Khan RS, et al. Differentiating drusen: Drusen
and drusen-like appearances associated with ageing, age-related
macular degeneration, inherited eye disease and other pathological
processes[ J]. Prog Retin Eye Res, 2016, 53: 70-106. DOI: 10.1016/
j.preteyeres.2016.04.008.Khan KN, Mahroo OA, Khan RS, et al. Differentiating drusen: Drusen
and drusen-like appearances associated with ageing, age-related
macular degeneration, inherited eye disease and other pathological
processes[ J]. Prog Retin Eye Res, 2016, 53: 70-106. DOI: 10.1016/
j.preteyeres.2016.04.008.
16、Goh KL, Chen FK, Balaratnasingam C, et al. Cuticular drusen in age�related macular degeneration: association with progression and impact on visual sensitivity[ J]. Ophthalmology, 2022, 129(6): 653-660. DOI:
10.1016/j.ophtha.2022.01.028.Goh KL, Chen FK, Balaratnasingam C, et al. Cuticular drusen in age�related macular degeneration: association with progression and impact on visual sensitivity[ J]. Ophthalmology, 2022, 129(6): 653-660. DOI:
10.1016/j.ophtha.2022.01.028.
17、Cheung R, Trinh M, Tee YG, et al. RPE curvature can screen for early
and intermediate AMD[ J]. Invest Ophthalmol Vis Sci, 2024, 65(2): 2.
DOI: 10.1167/iovs.65.2.2.Cheung R, Trinh M, Tee YG, et al. RPE curvature can screen for early
and intermediate AMD[ J]. Invest Ophthalmol Vis Sci, 2024, 65(2): 2.
DOI: 10.1167/iovs.65.2.2.
18、Kaufmann M, Han Z. RPE melanin and its influence on the progression
of AMD[ J]. Ageing Res Rev, 2024, 99: 102358. DOI: 10.1016/
j.arr.2024.102358.Kaufmann M, Han Z. RPE melanin and its influence on the progression
of AMD[ J]. Ageing Res Rev, 2024, 99: 102358. DOI: 10.1016/
j.arr.2024.102358.
19、Nashine S, Nesburn AB, Kuppermann BD, et al. Role of resveratrol in
transmitochondrial AMD RPE cells[ J]. Nutrients, 2020, 12(1): 159.
DOI: 10.3390/nu12010159.Nashine S, Nesburn AB, Kuppermann BD, et al. Role of resveratrol in
transmitochondrial AMD RPE cells[ J]. Nutrients, 2020, 12(1): 159.
DOI: 10.3390/nu12010159.
20、Ban N, Shinojima A, Negishi K, et al. Drusen in AMD from the
perspective of cholesterol metabolism and hypoxic response[ J]. J Clin
Med, 2024, 13(9): 2608. DOI: 10.3390/jcm13092608.Ban N, Shinojima A, Negishi K, et al. Drusen in AMD from the
perspective of cholesterol metabolism and hypoxic response[ J]. J Clin
Med, 2024, 13(9): 2608. DOI: 10.3390/jcm13092608.
21、Au A, Santina A, Abraham N, et al. Relationship between drusen height
and OCT biomarkers of atrophy in non-neovascular AMD[ J]. Invest
Ophthalmol Vis Sci, 2022, 63(11): 24. DOI: 10.1167/iovs.63.11.24.Au A, Santina A, Abraham N, et al. Relationship between drusen height
and OCT biomarkers of atrophy in non-neovascular AMD[ J]. Invest
Ophthalmol Vis Sci, 2022, 63(11): 24. DOI: 10.1167/iovs.63.11.24.
22、Colijn JM, den Hollander AI, Demirkan A, et al. Increased high-density
lipoprotein levels associated with age-related macular degeneration:
evidence from the EYE-RISK and European eye epidemiology
consortia[ J]. Ophthalmology, 2019, 126(3): 393-406. DOI: 10.1016/
j.ophtha.2018.09.045.Colijn JM, den Hollander AI, Demirkan A, et al. Increased high-density
lipoprotein levels associated with age-related macular degeneration:
evidence from the EYE-RISK and European eye epidemiology
consortia[ J]. Ophthalmology, 2019, 126(3): 393-406. DOI: 10.1016/
j.ophtha.2018.09.045.
23、Keenan TDL, Cukras CA, Chew EY. Age-related macular degeneration:
epidemiology and clinical aspects[M]//Advances in Experimental
Medicine and Biology. Cham: Springer International Publishing, 2021:
1-31. DOI: 10.1007/978-3-030-66014-7_1.Keenan TDL, Cukras CA, Chew EY. Age-related macular degeneration:
epidemiology and clinical aspects[M]//Advances in Experimental
Medicine and Biology. Cham: Springer International Publishing, 2021:
1-31. DOI: 10.1007/978-3-030-66014-7_1.
24、Jürgens F, Rothaus K, Faatz H, et al. Quantification of early and
intermediate age-related macular degeneration using OCT “en face”
presentation[ J]. Klin Monbl Augenheilkd, 2022, 239(1): 79-85. DOI:
10.1055/a-1327-3633.Jürgens F, Rothaus K, Faatz H, et al. Quantification of early and
intermediate age-related macular degeneration using OCT “en face”
presentation[ J]. Klin Monbl Augenheilkd, 2022, 239(1): 79-85. DOI:
10.1055/a-1327-3633.
25、Domínguez C, Heras J, Mata E, et al. Binary and multi-class automated
detection of age-related macular degeneration using convolutional�and transformer-based architectures[ J]. Comput Methods Programs
Biomed, 2023, 229: 107302. DOI: 10.1016/j.cmpb.2022.107302.Domínguez C, Heras J, Mata E, et al. Binary and multi-class automated
detection of age-related macular degeneration using convolutional�and transformer-based architectures[ J]. Comput Methods Programs
Biomed, 2023, 229: 107302. DOI: 10.1016/j.cmpb.2022.107302.
26、Boyce M, Xin Y, Chowdhury O, et al. Microglia-neutrophil interactions
drive dry AMD-like pathology in a mouse model[ J]. Cells, 2022,
11(22): 3535. DOI: 10.3390/cells11223535.Boyce M, Xin Y, Chowdhury O, et al. Microglia-neutrophil interactions
drive dry AMD-like pathology in a mouse model[ J]. Cells, 2022,
11(22): 3535. DOI: 10.3390/cells11223535.
27、Hudson N, Cahill M, Campbell M. Inner blood-retina barrier
involvement in dr y age-related macular degeneration (AMD)
pathology[ J]. Neural Regen Res, 2020, 15(9): 1656-1657. DOI:
10.4103/1673-5374.276332.Hudson N, Cahill M, Campbell M. Inner blood-retina barrier
involvement in dr y age-related macular degeneration (AMD)
pathology[ J]. Neural Regen Res, 2020, 15(9): 1656-1657. DOI:
10.4103/1673-5374.276332.
28、R amkumar HL, Zhang J, Chan CC. Retinal ultrastructure of
murine models of dry age-related macular degeneration (AMD)
[ J]. Prog Retin Eye Res, 2010, 29(3): 169-190. DOI: 10.1016/
j.preteyeres.2010.02.002.R amkumar HL, Zhang J, Chan CC. Retinal ultrastructure of
murine models of dry age-related macular degeneration (AMD)
[ J]. Prog Retin Eye Res, 2010, 29(3): 169-190. DOI: 10.1016/
j.preteyeres.2010.02.002.
29、López-Otín C, Blasco MA, Partridge L, et al., Hallmarks of aging: an
expanding universe[ J]. Cell, 2023. 186(2): 243-278. DOI: 10.1016/
j.cell.2022.11.001.López-Otín C, Blasco MA, Partridge L, et al., Hallmarks of aging: an
expanding universe[ J]. Cell, 2023. 186(2): 243-278. DOI: 10.1016/
j.cell.2022.11.001.
30、Baker DJ, Petersen RC. Cellular senescence in brain aging and
neurodegenerative diseases: evidence and perspectives[ J]. J Clin Invest,
2018, 128(4): 1208-1216. DOI: 10.1172/JCI95145.Baker DJ, Petersen RC. Cellular senescence in brain aging and
neurodegenerative diseases: evidence and perspectives[ J]. J Clin Invest,
2018, 128(4): 1208-1216. DOI: 10.1172/JCI95145.
31、de Luzy IR , Lee MK, Mobley WC, et al. Lessons from inducible
pluripotent stem cell models on neuronal senescence in aging and
neurodegeneration[ J]. Nat Aging, 2024, 4(3): 309-318. DOI:
10.1038/s43587-024-00586-3.de Luzy IR , Lee MK, Mobley WC, et al. Lessons from inducible
pluripotent stem cell models on neuronal senescence in aging and
neurodegeneration[ J]. Nat Aging, 2024, 4(3): 309-318. DOI:
10.1038/s43587-024-00586-3.
32、Zhang W, Sun HS, Wang X, et al. Cellular senescence, DNA damage,
and neuroinflammation in the aging brain[ J]. Trends Neurosci, 2024,
47(6): 461-474. DOI: 10.1016/j.tins.2024.04.003.Zhang W, Sun HS, Wang X, et al. Cellular senescence, DNA damage,
and neuroinflammation in the aging brain[ J]. Trends Neurosci, 2024,
47(6): 461-474. DOI: 10.1016/j.tins.2024.04.003.
33、Binder S, Stanzel BV, Krebs I, et al. Transplantation of the RPE in
AMD[ J]. Prog Retin Eye Res, 2007, 26(5): 516-554. DOI: 10.1016/
j.preteyeres.2007.02.002.Binder S, Stanzel BV, Krebs I, et al. Transplantation of the RPE in
AMD[ J]. Prog Retin Eye Res, 2007, 26(5): 516-554. DOI: 10.1016/
j.preteyeres.2007.02.002.
34、Blasiak J, Sobczuk P, Pawlowska E, et al. Interplay between aging
and other factors of the pathogenesis of age-related macular
degeneration[ J]. Ageing Res Rev, 2022, 81: 101735. DOI: 10.1016/
j.arr.2022.101735.Blasiak J, Sobczuk P, Pawlowska E, et al. Interplay between aging
and other factors of the pathogenesis of age-related macular
degeneration[ J]. Ageing Res Rev, 2022, 81: 101735. DOI: 10.1016/
j.arr.2022.101735.
35、Upadhyay M, Milliner C, Bell BA, et al. Oxidative stress in the retina
and retinal pigment epithelium (RPE): role of aging, and DJ-1[ J].
Redox Biol, 2020, 37: 101623. DOI: 10.1016/j.redox.2020.101623.Upadhyay M, Milliner C, Bell BA, et al. Oxidative stress in the retina
and retinal pigment epithelium (RPE): role of aging, and DJ-1[ J].
Redox Biol, 2020, 37: 101623. DOI: 10.1016/j.redox.2020.101623.
36、Hayflick L, Moorhead PS. The serial cultivation of human diploid cell
strains[ J]. Exp Cell Res, 1961, 25(3): 585-621. DOI: 10.1016/0014-
4827(61)90192-6.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell
strains[ J]. Exp Cell Res, 1961, 25(3): 585-621. DOI: 10.1016/0014-
4827(61)90192-6.
37、Li Y, Zhou G, Bruno IG, et al. Telomerase mRNA reverses senescence
in progeria cells[ J]. J Am Coll Cardiol, 2017, 70(6): 804-805. DOI:
10.1016/j.jacc.2017.06.017.Li Y, Zhou G, Bruno IG, et al. Telomerase mRNA reverses senescence
in progeria cells[ J]. J Am Coll Cardiol, 2017, 70(6): 804-805. DOI:
10.1016/j.jacc.2017.06.017.
38、Tang H, Geng A, Zhang T, et al. Single senescent cell sequencing
reveals heterogeneity in senescent cells induced by telomere erosion[ J].
Protein Cell, 2019, 10(5): 370-375. DOI: 10.1007/s13238-018-
0591-y.Tang H, Geng A, Zhang T, et al. Single senescent cell sequencing
reveals heterogeneity in senescent cells induced by telomere erosion[ J].
Protein Cell, 2019, 10(5): 370-375. DOI: 10.1007/s13238-018-
0591-y.
39、Vinciguerra M. Telomere oxidative lesions and cell senescence[ J]. Nat
Aging, 2022, 2: 690. DOI: 10.1038/s43587-022-00272-2.Vinciguerra M. Telomere oxidative lesions and cell senescence[ J]. Nat
Aging, 2022, 2: 690. DOI: 10.1038/s43587-022-00272-2.
40、Vaddavalli PL, Schumacher B. The p53 network: cellular and systemic
DNA damage responses in cancer and aging[ J]. Trends Genet, 2022,
38(6): 598-612. DOI: 10.1016/j.tig.2022.02.010.Vaddavalli PL, Schumacher B. The p53 network: cellular and systemic
DNA damage responses in cancer and aging[ J]. Trends Genet, 2022,
38(6): 598-612. DOI: 10.1016/j.tig.2022.02.010.
41、Sturmlechner I, Sine CC, Jeganathan KB, et al. Senescent cells limit p53
activity via multiple mechanisms to remain viable[ J]. Nat Commun,
2022, 13(1): 3722. DOI: 10.1038/s41467-022-31239-x.Sturmlechner I, Sine CC, Jeganathan KB, et al. Senescent cells limit p53
activity via multiple mechanisms to remain viable[ J]. Nat Commun,
2022, 13(1): 3722. DOI: 10.1038/s41467-022-31239-x.
42、Peng Y, Du J, Günther S, et al. Mechano-signaling via Piezo1 prevents
activation and p53-mediated senescence of muscle stem cells[ J]. Redox
Biol, 2022, 52: 102309. DOI: 10.1016/j.redox.2022.102309.Peng Y, Du J, Günther S, et al. Mechano-signaling via Piezo1 prevents
activation and p53-mediated senescence of muscle stem cells[ J]. Redox
Biol, 2022, 52: 102309. DOI: 10.1016/j.redox.2022.102309.
43、Zhong Y, Wang G, Yang S, et al. The role of DNA damage in neural stem
cells ageing[ J]. J Cell Physiol, 2024, 239(4): e31187. DOI: 10.1002/
jcp.31187.Zhong Y, Wang G, Yang S, et al. The role of DNA damage in neural stem
cells ageing[ J]. J Cell Physiol, 2024, 239(4): e31187. DOI: 10.1002/
jcp.31187.
44、Cheung P, Vallania F, Warsinske HC, et al. Single-cell chromatin
modification profiling reveals increased epigenetic variations with
aging[ J]. Cell, 2018, 173(6): 1385-1397.e14. DOI: 10.1016/
j.cell.2018.03.079.Cheung P, Vallania F, Warsinske HC, et al. Single-cell chromatin
modification profiling reveals increased epigenetic variations with
aging[ J]. Cell, 2018, 173(6): 1385-1397.e14. DOI: 10.1016/
j.cell.2018.03.079.
45、Sanders YY, Liu H, Zhang X, et al. Histone modifications in senescence�associated resistance to apoptosis by oxidative stress[ J]. Redox Biol,
2013, 1(1): 8-16. DOI: 10.1016/j.redox.2012.11.004.Sanders YY, Liu H, Zhang X, et al. Histone modifications in senescence�associated resistance to apoptosis by oxidative stress[ J]. Redox Biol,
2013, 1(1): 8-16. DOI: 10.1016/j.redox.2012.11.004.
46、Mei Q, Xu C, Gogol M, et al. Set1-catalyzed H3K4 trimethylation
antagonizes the HIR/Asf1/Rtt106 repressor complex to promote
histone gene expression and chronological life span[ J]. Nucleic Acids
Res, 2019, 47(7): 3434-3449. DOI: 10.1093/nar/gkz101.Mei Q, Xu C, Gogol M, et al. Set1-catalyzed H3K4 trimethylation
antagonizes the HIR/Asf1/Rtt106 repressor complex to promote
histone gene expression and chronological life span[ J]. Nucleic Acids
Res, 2019, 47(7): 3434-3449. DOI: 10.1093/nar/gkz101.
47、Yang L, Ma Z, Wang H, et al. Ubiquitylome study identifies increased
histone 2A ubiquitylation as an evolutionarily conserved aging
biomarker[ J]. Nat Commun, 2019, 10(1): 2191. DOI: 10.1038/
s41467-019-10136-w.Yang L, Ma Z, Wang H, et al. Ubiquitylome study identifies increased
histone 2A ubiquitylation as an evolutionarily conserved aging
biomarker[ J]. Nat Commun, 2019, 10(1): 2191. DOI: 10.1038/
s41467-019-10136-w.
48、Place RF, Noonan EJ, Giardina C. HDACs and the senescent phenotype
of WI-38 cells[ J]. BMC Cell Biol, 2005, 6: 37. DOI: 10.1186/1471-
2121-6-37.Place RF, Noonan EJ, Giardina C. HDACs and the senescent phenotype
of WI-38 cells[ J]. BMC Cell Biol, 2005, 6: 37. DOI: 10.1186/1471-
2121-6-37.
49、Warnon C, Bouhjar K, Ninane N, et al. HDAC2 and 7 down-regulation
induces senescence in dermal fibroblasts[ J]. Aging, 2021, 13(14):
17978-18005. DOI: 10.18632/aging.203304.Warnon C, Bouhjar K, Ninane N, et al. HDAC2 and 7 down-regulation
induces senescence in dermal fibroblasts[ J]. Aging, 2021, 13(14):
17978-18005. DOI: 10.18632/aging.203304.
50、Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline
in ageing[ J]. Nat Rev Mol Cell Biol, 2019, 20: 421-435. DOI: 10.1038/
s41580-019-0101-y.Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline
in ageing[ J]. Nat Rev Mol Cell Biol, 2019, 20: 421-435. DOI: 10.1038/
s41580-019-0101-y.
51、Bakalova R , Aoki I, Zhelev Z, et al. Cellular redox imbalance on
the crossroad between mitochondrial dysfunction, senescence, and
proliferation[ J]. Redox Biol, 2022, 53: 102337. DOI: 10.1016/
j.redox.2022.102337.Bakalova R , Aoki I, Zhelev Z, et al. Cellular redox imbalance on
the crossroad between mitochondrial dysfunction, senescence, and
proliferation[ J]. Redox Biol, 2022, 53: 102337. DOI: 10.1016/
j.redox.2022.102337.
52、Byrns CN, Perlegos AE, Miller KN, et al. Senescent glia link
mitochondrial dysfunction and lipid accumulation[ J]. Nature, 2024,
630(8016): 475-483. DOI: 10.1038/s41586-024-07516-8.Byrns CN, Perlegos AE, Miller KN, et al. Senescent glia link
mitochondrial dysfunction and lipid accumulation[ J]. Nature, 2024,
630(8016): 475-483. DOI: 10.1038/s41586-024-07516-8.
53、Miwa S, Kashyap S, Chini E, et al. Mitochondrial dysfunction in cell
senescence and aging[ J]. J Clin Invest, 2022, 132(13): e158447. DOI:
10.1172/JCI158447.Miwa S, Kashyap S, Chini E, et al. Mitochondrial dysfunction in cell
senescence and aging[ J]. J Clin Invest, 2022, 132(13): e158447. DOI:
10.1172/JCI158447.
54、Faget DV, Ren Q, Stewart SA. Unmasking senescence: context�dependent effects of SASP in cancer[ J]. Nat Rev Cancer, 2019, 19(8):
439-453. DOI: 10.1038/s41568-019-0156-2.Faget DV, Ren Q, Stewart SA. Unmasking senescence: context�dependent effects of SASP in cancer[ J]. Nat Rev Cancer, 2019, 19(8):
439-453. DOI: 10.1038/s41568-019-0156-2.
55、Victorelli S, Salmonowicz H, Chapman J, et al. Apoptotic stress causes
mtDNA release during senescence and drives the SASP[ J]. Nature,
2023, 622(7983): 627-636. DOI: 10.1038/s41586-023-06621-4.Victorelli S, Salmonowicz H, Chapman J, et al. Apoptotic stress causes
mtDNA release during senescence and drives the SASP[ J]. Nature,
2023, 622(7983): 627-636. DOI: 10.1038/s41586-023-06621-4.
56、Ito Y, Hoare M, Nar ita M. Spatial and temporal control of
senescence[ J]. Trends Cell Biol, 2017, 27(11): 820-832. DOI:
10.1016/j.tcb.2017.07.004.Ito Y, Hoare M, Nar ita M. Spatial and temporal control of
senescence[ J]. Trends Cell Biol, 2017, 27(11): 820-832. DOI:
10.1016/j.tcb.2017.07.004.
57、Conte TC, Duran-Bishop G, Orfi Z, et al. Clearance of defective muscle
stem cells by senolytics restores myogenesis in myotonic dystrophy
type 1[ J]. Nat Commun, 2023, 14(1): 4033. DOI: 10.1038/s41467-
023-39663-3.Conte TC, Duran-Bishop G, Orfi Z, et al. Clearance of defective muscle
stem cells by senolytics restores myogenesis in myotonic dystrophy
type 1[ J]. Nat Commun, 2023, 14(1): 4033. DOI: 10.1038/s41467-
023-39663-3.
58、Lee BY, Han JA, Im JS, et al. Senescence-associated beta-galactosidase
is lysosomal beta-galactosidase[ J]. Aging Cell, 2006, 5(2): 187-195.
DOI: 10.1111/j.1474-9726.2006.00199.x.Lee BY, Han JA, Im JS, et al. Senescence-associated beta-galactosidase
is lysosomal beta-galactosidase[ J]. Aging Cell, 2006, 5(2): 187-195.
DOI: 10.1111/j.1474-9726.2006.00199.x.
59、Malek G, Li CM, Guidry C, et al. Apolipoprotein B in cholesterol�containing drusen and basal deposits of human eyes with age-related
maculopathy[ J]. Am J Pathol, 2003, 162(2): 413-425. DOI: 10.1016/
s0002-9440(10)63836-9.Malek G, Li CM, Guidry C, et al. Apolipoprotein B in cholesterol�containing drusen and basal deposits of human eyes with age-related
maculopathy[ J]. Am J Pathol, 2003, 162(2): 413-425. DOI: 10.1016/
s0002-9440(10)63836-9.
60、Curcio CA, Johnson M, Rudolf M, et al. The oil spill in ageing Bruch
membrane[ J]. Br J Ophthalmol, 2011,95(12):1638-1645. DOI:
10.1136/bjophthalmol-2011-300344.Curcio CA, Johnson M, Rudolf M, et al. The oil spill in ageing Bruch
membrane[ J]. Br J Ophthalmol, 2011,95(12):1638-1645. DOI:
10.1136/bjophthalmol-2011-300344.
61、Alves CH, Fernandes R, Santiago AR, etal. Microglia contribution to
the regulation of the retinal and choroidal vasculature in age-related
macular degeneration[ J]. Cells, 2020,9(5):1217. DOI:10.3390/
cells9051217.Alves CH, Fernandes R, Santiago AR, etal. Microglia contribution to
the regulation of the retinal and choroidal vasculature in age-related
macular degeneration[ J]. Cells, 2020,9(5):1217. DOI:10.3390/
cells9051217.
62、Cabrera AP, Bhaskaran A, Xu J, et al. Senescence increases choroidal
endothelial stiffness and susceptibility to complement injury:
implications for choriocapillaris loss in AMD[ J]. Invest Ophthalmol
Vis Sci, 2016, 57(14): 5910-5918. DOI: 10.1167/iovs.16-19727.Cabrera AP, Bhaskaran A, Xu J, et al. Senescence increases choroidal
endothelial stiffness and susceptibility to complement injury:
implications for choriocapillaris loss in AMD[ J]. Invest Ophthalmol
Vis Sci, 2016, 57(14): 5910-5918. DOI: 10.1167/iovs.16-19727.
63、Damani MR, Zhao L, Fontainhas AM, et al. Age-related alterations in
the dynamic behavior of microglia[ J]. Aging Cell, 2011, 10(2): 263-
276. DOI: 10.1111/j.1474-9726.2010.00660.x.Damani MR, Zhao L, Fontainhas AM, et al. Age-related alterations in
the dynamic behavior of microglia[ J]. Aging Cell, 2011, 10(2): 263-
276. DOI: 10.1111/j.1474-9726.2010.00660.x.
64、Kozlowski MR. RPE cell senescence: a key contributor to age-related
macular degeneration[ J]. Med Hypotheses, 2012, 78(4): 505-510.
DOI: 10.1016/j.mehy.2012.01.018.Kozlowski MR. RPE cell senescence: a key contributor to age-related
macular degeneration[ J]. Med Hypotheses, 2012, 78(4): 505-510.
DOI: 10.1016/j.mehy.2012.01.018.
65、Ouyang X, Yang J, Hong Z, et al. Mechanisms of blue light-induced eye
hazard and protective measures: a review[ J]. Biomed Pharmacother.
2020;130:110577. DOI:10.1016/j.biopha.2020.110577.Ouyang X, Yang J, Hong Z, et al. Mechanisms of blue light-induced eye
hazard and protective measures: a review[ J]. Biomed Pharmacother.
2020;130:110577. DOI:10.1016/j.biopha.2020.110577.
66、Zhu X, Liu W, Tang X, et al. The BET PROTAC inhibitor dBET6
protects against retinal degeneration and inhibits the cGAS-STING in
response to light damage[ J]. J Neuroinflammation, 2023, 20(1): 119.
DOI: 10.1186/s12974-023-02804-y.Zhu X, Liu W, Tang X, et al. The BET PROTAC inhibitor dBET6
protects against retinal degeneration and inhibits the cGAS-STING in
response to light damage[ J]. J Neuroinflammation, 2023, 20(1): 119.
DOI: 10.1186/s12974-023-02804-y.
67、Zou M, Ke Q, Nie Q, et al., Inhibition of cGAS-STING by JQ1
allev iates ox idative stress-induced retina inflammation and
degeneration[ J]. Cell Death Differ, 2022, 29(9): 1816-1833. DOI:
10.1038/s41418-022-00967-4.Zou M, Ke Q, Nie Q, et al., Inhibition of cGAS-STING by JQ1
allev iates ox idative stress-induced retina inflammation and
degeneration[ J]. Cell Death Differ, 2022, 29(9): 1816-1833. DOI:
10.1038/s41418-022-00967-4.
68、Kozhevnikova OS, Korbolina EE, Ershov NI, et al. Rat retinal
transcriptome: effects of aging and AMD-like retinopathy[ J]. Cell
Cycle Georget Tex, 2013, 12(11): 1745-1761. DOI: 10.4161/cc.24825.Kozhevnikova OS, Korbolina EE, Ershov NI, et al. Rat retinal
transcriptome: effects of aging and AMD-like retinopathy[ J]. Cell
Cycle Georget Tex, 2013, 12(11): 1745-1761. DOI: 10.4161/cc.24825.
69、Kaarniranta K, Tokarz P, Koskela A, et al. Autophagy regulates death of
retinal pigment epithelium cells in age-related macular degeneration[ J].
Cell Biol Toxicol, 2017, 33(2): 113-128. DOI: 10.1007/s10565-016-9371-8.Kaarniranta K, Tokarz P, Koskela A, et al. Autophagy regulates death of
retinal pigment epithelium cells in age-related macular degeneration[ J].
Cell Biol Toxicol, 2017, 33(2): 113-128. DOI: 10.1007/s10565-016-9371-8.
70、Gupta U, Ghosh S, Wallace CT, et al. Increased LCN2 (lipocalin 2) in
the RPE decreases autophagy and activates inflammasome-ferroptosis
processes in a mouse model of dry AMD[ J]. Autophagy, 2023, 19(1):
92-111. DOI: 10.1080/15548627.2022.2062887.Gupta U, Ghosh S, Wallace CT, et al. Increased LCN2 (lipocalin 2) in
the RPE decreases autophagy and activates inflammasome-ferroptosis
processes in a mouse model of dry AMD[ J]. Autophagy, 2023, 19(1):
92-111. DOI: 10.1080/15548627.2022.2062887.
71、Nita M, Grzybowski A. Antioxidative role of heterophagy, autophagy,
and mitophagy in the retina and their association with the age-related
macular degeneration (AMD) etiopathogenesis[ J]. Antioxidants, 2023,
12(7): 1368. DOI: 10.3390/antiox12071368.Nita M, Grzybowski A. Antioxidative role of heterophagy, autophagy,
and mitophagy in the retina and their association with the age-related
macular degeneration (AMD) etiopathogenesis[ J]. Antioxidants, 2023,
12(7): 1368. DOI: 10.3390/antiox12071368.
72、Golestaneh N, Chu Y, Xiao YY, et al. Dysfunctional autophagy in RPE,
a contributing factor in age-related macular degeneration[ J]. Cell
Death Dis, 2017, 8(1): e2537. DOI: 10.1038/cddis.2016.453.Golestaneh N, Chu Y, Xiao YY, et al. Dysfunctional autophagy in RPE,
a contributing factor in age-related macular degeneration[ J]. Cell
Death Dis, 2017, 8(1): e2537. DOI: 10.1038/cddis.2016.453.
73、Mitter SK, Song C, Qi X, et al. Dysregulated autophagy in the RPE is
associated with increased susceptibility to oxidative stress and AMD[ J].
Autophagy, 2014, 10(11): 1989-2005. DOI: 10.4161/auto.36184.Mitter SK, Song C, Qi X, et al. Dysregulated autophagy in the RPE is
associated with increased susceptibility to oxidative stress and AMD[ J].
Autophagy, 2014, 10(11): 1989-2005. DOI: 10.4161/auto.36184.
74、Datta S, Cano M, Satyanarayana G, et al. Mitophagy initiates retrograde
mitochondrial-nuclear signaling to guide retinal pigment cell
heterogeneity[ J]. Autophagy,2023,19(3):966-983. DOI:10.1080/155
48627.2022.2109286.Datta S, Cano M, Satyanarayana G, et al. Mitophagy initiates retrograde
mitochondrial-nuclear signaling to guide retinal pigment cell
heterogeneity[ J]. Autophagy,2023,19(3):966-983. DOI:10.1080/155
48627.2022.2109286.
75、Abokyi S, Shan SW, Lam CH, et al. Targeting lysosomes to reverse
hydroquinone-induced autophagy defects and oxidative damage in
human retinal pigment epithelial cells[ J]. Int J Mol Sci, 2021, 22(16):
9042. DOI: 10.3390/ijms22169042.Abokyi S, Shan SW, Lam CH, et al. Targeting lysosomes to reverse
hydroquinone-induced autophagy defects and oxidative damage in
human retinal pigment epithelial cells[ J]. Int J Mol Sci, 2021, 22(16):
9042. DOI: 10.3390/ijms22169042.
76、Chen X, Zhu Y, Shi X, et al. Ming-mu-di-Huang-pill activates SQSTM1
via AMPK-mediated autophagic KEAP1 degradation and protects RPE
cells from oxidative damage[ J]. Oxid Med Cell Longev, 2022, 2022:
5851315. DOI: 10.1155/2022/5851315.Chen X, Zhu Y, Shi X, et al. Ming-mu-di-Huang-pill activates SQSTM1
via AMPK-mediated autophagic KEAP1 degradation and protects RPE
cells from oxidative damage[ J]. Oxid Med Cell Longev, 2022, 2022:
5851315. DOI: 10.1155/2022/5851315.
77、Ferreira Franco E, Rocha J, Carvalho H, et al. An analysis of technical
debt management through resources allocation policies in software
maintenance process[ J]. arXiv E Prints, 2016: arXiv: 1609.06868.
DOI: 10.48550/arXiv.1609.06868.Ferreira Franco E, Rocha J, Carvalho H, et al. An analysis of technical
debt management through resources allocation policies in software
maintenance process[ J]. arXiv E Prints, 2016: arXiv: 1609.06868.
DOI: 10.48550/arXiv.1609.06868.
78、Lakkaraju A, Umapathy A, Tan LX, et al. The cell biology of the retinal
pigment epithelium[ J]. Prog Retin Eye Res, 2020: 100846. DOI:
10.1016/j.preteyeres.2020.100846.Lakkaraju A, Umapathy A, Tan LX, et al. The cell biology of the retinal
pigment epithelium[ J]. Prog Retin Eye Res, 2020: 100846. DOI:
10.1016/j.preteyeres.2020.100846.
79、Hyttinen%20JMT%2C%20B%C5%82asiak%20J%2C%20Niittykoski%20M%2C%20et%20al.%20DNA%20damage%20response%20%0Aand%20autophagy%20in%20the%20degeneration%20of%20retinal%20pigment%20epithelial%20cells%02Implications%20for%20age-related%20macular%20degeneration%20(AMD)%5B%20J%5D.%20Ageing%20%0ARes%20Rev%2C%202017%2C%2036%3A%2064-77.%20DOI%3A%2010.1016%2Fj.arr.2017.03.006.Hyttinen%20JMT%2C%20B%C5%82asiak%20J%2C%20Niittykoski%20M%2C%20et%20al.%20DNA%20damage%20response%20%0Aand%20autophagy%20in%20the%20degeneration%20of%20retinal%20pigment%20epithelial%20cells%02Implications%20for%20age-related%20macular%20degeneration%20(AMD)%5B%20J%5D.%20Ageing%20%0ARes%20Rev%2C%202017%2C%2036%3A%2064-77.%20DOI%3A%2010.1016%2Fj.arr.2017.03.006.
80、Fields MA, del Priore LV, Adelman RA, et al. Interactions of the
choroid, Bruch's membrane, retinal pigment epithelium, and
neurosensory retina collaborate to form the outer blood-retinal�barrier[ J]. Prog Retin Eye Res, 2020, 76: 100803. DOI: 10.1016/
j.preteyeres.2019.100803.Fields MA, del Priore LV, Adelman RA, et al. Interactions of the
choroid, Bruch's membrane, retinal pigment epithelium, and
neurosensory retina collaborate to form the outer blood-retinal�barrier[ J]. Prog Retin Eye Res, 2020, 76: 100803. DOI: 10.1016/
j.preteyeres.2019.100803.
81、Naylor A, Hopkins A, Hudson N, et al. Tight junctions of the outer
blood retina barrier[ J]. Int J Mol Sci, 2019, 21(1): 211. DOI: 10.3390/ijms21010211.Naylor A, Hopkins A, Hudson N, et al. Tight junctions of the outer
blood retina barrier[ J]. Int J Mol Sci, 2019, 21(1): 211. DOI: 10.3390/ijms21010211.
82、Xia T, Rizzolo LJ. Effects of diabetic retinopathy on the barrier
functions of the retinal pigment epithelium[ J]. Vision Res, 2017, 139:
72-81. DOI: 10.1016/j.visres.2017.02.006.Xia T, Rizzolo LJ. Effects of diabetic retinopathy on the barrier
functions of the retinal pigment epithelium[ J]. Vision Res, 2017, 139:
72-81. DOI: 10.1016/j.visres.2017.02.006.
83、Datta S, Cano M, Ebrahimi K, et al. The impact of oxidative stress
and inflammation on RPE degeneration in non-neovascular
AMD[ J]. Prog Retin Eye Res, 2017, 60: 201-218. DOI: 10.1016/
j.preteyeres.2017.03.002.Datta S, Cano M, Ebrahimi K, et al. The impact of oxidative stress
and inflammation on RPE degeneration in non-neovascular
AMD[ J]. Prog Retin Eye Res, 2017, 60: 201-218. DOI: 10.1016/
j.preteyeres.2017.03.002.
84、Park SE, Song JD, Kim KM, et al. Diphenyleneiodonium induces
ROS-independent p53 expression and apoptosis in human RPE
cells[ J]. FEBS Lett, 2007, 581(2): 180-186. DOI: 10.1016/
j.febslet.2006.12.006.Park SE, Song JD, Kim KM, et al. Diphenyleneiodonium induces
ROS-independent p53 expression and apoptosis in human RPE
cells[ J]. FEBS Lett, 2007, 581(2): 180-186. DOI: 10.1016/
j.febslet.2006.12.006.
85、Zhang Y, Liu Y, Ho C, et al. Effects of imposed defocus of opposite
sign on temporal gene expression patterns of BMP4 and BMP7 in
chick RPE[ J]. Exp Eye Res, 2013, 109: 98-106. DOI: 10.1016/
j.exer.2013.02.010.Zhang Y, Liu Y, Ho C, et al. Effects of imposed defocus of opposite
sign on temporal gene expression patterns of BMP4 and BMP7 in
chick RPE[ J]. Exp Eye Res, 2013, 109: 98-106. DOI: 10.1016/
j.exer.2013.02.010.
86、Gregory CY, Hall MO. The phagocytosis of ROS by RPE cells is
inhibited by an antiserum to rat RPE cell plasma membranes[ J]. Exp
Eye Res, 1992, 54(6): 843-851. DOI: 10.1016/0014-4835(92)90147-
k.Gregory CY, Hall MO. The phagocytosis of ROS by RPE cells is
inhibited by an antiserum to rat RPE cell plasma membranes[ J]. Exp
Eye Res, 1992, 54(6): 843-851. DOI: 10.1016/0014-4835(92)90147-
k.
87、Hall MO, Abrams TA. RPE cells from normal rats do not secrete a
factor which enhances the phagocytosis of ROS by dystrophic rat RPE
cells[ J]. Exp Eye Res, 1991, 52(4): 461-464. DOI: 10.1016/0014-
4835(91)90043-e.Hall MO, Abrams TA. RPE cells from normal rats do not secrete a
factor which enhances the phagocytosis of ROS by dystrophic rat RPE
cells[ J]. Exp Eye Res, 1991, 52(4): 461-464. DOI: 10.1016/0014-
4835(91)90043-e.
88、Tong Y, Wu Y, Ma J, et al. Comparative mechanistic study of
RPE cell death induced by different oxidative stresses[ J]. Redox
Biol,2023,65:102840. DOI:10.1016/j.redox.2023.102840.Tong Y, Wu Y, Ma J, et al. Comparative mechanistic study of
RPE cell death induced by different oxidative stresses[ J]. Redox
Biol,2023,65:102840. DOI:10.1016/j.redox.2023.102840.
89、Ferrington DA, Kapphahn RJ, Leary MM, et al. Increased retinal
mtDNA damage in the CFH variant associated with age-related macular
degeneration[ J]. Exp Eye Res, 2016, 145: 269-277. DOI: 10.1016/
j.exer.2016.01.018.Ferrington DA, Kapphahn RJ, Leary MM, et al. Increased retinal
mtDNA damage in the CFH variant associated with age-related macular
degeneration[ J]. Exp Eye Res, 2016, 145: 269-277. DOI: 10.1016/
j.exer.2016.01.018.
90、K aarniranta K , Uusitalo H, Blasiak J, et al. Mechanisms of
mitochondrial dysfunction and their impact on age-related macular
degeneration[ J]. Prog Retin Eye Res, 2020, 79: 100858. DOI:
10.1016/j.preteyeres.2020.100858.K aarniranta K , Uusitalo H, Blasiak J, et al. Mechanisms of
mitochondrial dysfunction and their impact on age-related macular
degeneration[ J]. Prog Retin Eye Res, 2020, 79: 100858. DOI:
10.1016/j.preteyeres.2020.100858.
91、Panvini AR, Gvritishvili A, Galvan H, et al. Differential mitochondrial
and cellular responses between H vs. J mtDNA haplogroup-containing
human RPE transmitochondrial cybrid cells[ J]. Exp Eye Res, 2022,
219: 109013. DOI: 10.1016/j.exer.2022.109013.Panvini AR, Gvritishvili A, Galvan H, et al. Differential mitochondrial
and cellular responses between H vs. J mtDNA haplogroup-containing
human RPE transmitochondrial cybrid cells[ J]. Exp Eye Res, 2022,
219: 109013. DOI: 10.1016/j.exer.2022.109013.
92、Saada J, McAuley RJ, Marcatti M, et al. Oxidative stress induces
Z-DNA-binding protein 1-dependent activation of microglia via
mtDNA released from retinal pigment epithelial cells[ J]. J Biol Chem,
2022, 298(1): 101523. DOI: 10.1016/j.jbc.2021.101523.Saada J, McAuley RJ, Marcatti M, et al. Oxidative stress induces
Z-DNA-binding protein 1-dependent activation of microglia via
mtDNA released from retinal pigment epithelial cells[ J]. J Biol Chem,
2022, 298(1): 101523. DOI: 10.1016/j.jbc.2021.101523.
93、Brown EE, DeWeerd AJ, Ildefonso CJ, et al. Mitochondrial oxidative
stress in the retinal pigment epithelium (RPE) led to metabolic
dysfunction in both the RPE and retinal photoreceptors[ J]. Redox
Biol, 2019, 24: 101201. DOI: 10.1016/j.redox.2019.101201.Brown EE, DeWeerd AJ, Ildefonso CJ, et al. Mitochondrial oxidative
stress in the retinal pigment epithelium (RPE) led to metabolic
dysfunction in both the RPE and retinal photoreceptors[ J]. Redox
Biol, 2019, 24: 101201. DOI: 10.1016/j.redox.2019.101201.
94、Lin H, Xu H, Liang FQ, et al. Mitochondrial DNA damage and repair
in RPE associated with aging and age-related macular degeneration[ J].
Invest Ophthalmol Vis Sci,2011,52(6):3521-3529. DOI:10.1167/
iovs.10-6163.Lin H, Xu H, Liang FQ, et al. Mitochondrial DNA damage and repair
in RPE associated with aging and age-related macular degeneration[ J].
Invest Ophthalmol Vis Sci,2011,52(6):3521-3529. DOI:10.1167/
iovs.10-6163.
95、Tong Y, Wu Y, Ma J, et al. Comparative mechanistic study of RPE cell
death induced by different oxidative stresses[ J]. Redox Biol, 2023, 65:
102840. DOI: 10.1016/j.redox.2023.102840.Tong Y, Wu Y, Ma J, et al. Comparative mechanistic study of RPE cell
death induced by different oxidative stresses[ J]. Redox Biol, 2023, 65:
102840. DOI: 10.1016/j.redox.2023.102840.
96、K atz ML, Drea CM, Robison WG Jr. Relationship bet ween
dietary retinol and lipofuscin in the retinal pigment epithelium[ J].
Mech Ageing Dev, 1986, 35(3): 291-305. DOI: 10.1016/0047-
6374(86)90131-4.K atz ML, Drea CM, Robison WG Jr. Relationship bet ween
dietary retinol and lipofuscin in the retinal pigment epithelium[ J].
Mech Ageing Dev, 1986, 35(3): 291-305. DOI: 10.1016/0047-
6374(86)90131-4.
97、Zhang M, Chu Y, Mowery J, et al. Pgc-1α repression and high-fat
diet induce age-related macular degeneration-like phenotypes in
mice[ J]. Dis Model Mech, 2018, 11(9): dmm032698. DOI: 10.1242/
dmm.032698.Zhang M, Chu Y, Mowery J, et al. Pgc-1α repression and high-fat
diet induce age-related macular degeneration-like phenotypes in
mice[ J]. Dis Model Mech, 2018, 11(9): dmm032698. DOI: 10.1242/
dmm.032698.
98、Kushwah N, Bora K , Maur ya M, et al.Ox idative stress and
antioxidants in age-related macular degeneration[ J].Antioxidants(Bas
el),2023,12(7):1379. DOI:10.3390/antiox12071379.Kushwah N, Bora K , Maur ya M, et al.Ox idative stress and
antioxidants in age-related macular degeneration[ J].Antioxidants(Bas
el),2023,12(7):1379. DOI:10.3390/antiox12071379.
99、Wang L, Kaya KD, Kim S, et al. Retinal pigment epithelium
transcriptome analysis in chronic smoking reveals a suppressed innate
immune response and activation of differentiation pathways[ J].
Free Radic Biol Med, 2020, 156: 176-189. DOI: 10.1016/
j.freeradbiomed.2020.06.004.Wang L, Kaya KD, Kim S, et al. Retinal pigment epithelium
transcriptome analysis in chronic smoking reveals a suppressed innate
immune response and activation of differentiation pathways[ J].
Free Radic Biol Med, 2020, 156: 176-189. DOI: 10.1016/
j.freeradbiomed.2020.06.004.
100、Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the
aging RPE given an oxidative insult[ J]. Exp Eye Res, 2014, 119: 111-
114. DOI: 10.1016/j.exer.2013.10.024.Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the
aging RPE given an oxidative insult[ J]. Exp Eye Res, 2014, 119: 111-
114. DOI: 10.1016/j.exer.2013.10.024.
101、Xu XZ, Tang Y, Cheng LB, et al. Targeting Keap1 by miR-626 protects
retinal pigment epithelium cells from oxidative injury by activating
Nrf2 signaling[ J]. Free Radic Biol Med, 2019, 143: 387-396. DOI:
10.1016/j.freeradbiomed.2019.08.024.Xu XZ, Tang Y, Cheng LB, et al. Targeting Keap1 by miR-626 protects
retinal pigment epithelium cells from oxidative injury by activating
Nrf2 signaling[ J]. Free Radic Biol Med, 2019, 143: 387-396. DOI:
10.1016/j.freeradbiomed.2019.08.024.
102、Yang J, Hua Z, Zheng Z, et al. Acteoside inhibits high glucose-induced
oxidative stress injury in RPE cells and the outer retina through the
Keap1/Nrf2/ARE pathway[ J]. Exp Eye Res, 2023, 232: 109496. DOI:
10.1016/j.exer.2023.109496.Yang J, Hua Z, Zheng Z, et al. Acteoside inhibits high glucose-induced
oxidative stress injury in RPE cells and the outer retina through the
Keap1/Nrf2/ARE pathway[ J]. Exp Eye Res, 2023, 232: 109496. DOI:
10.1016/j.exer.2023.109496.
103、Dvorkin S, Cambier S, Volkman HE, et al. New frontiers in the cGAS�STING intracellular DNA-sensing pathway[ J]. Immunity, 2024, 57(4):
718-730. DOI: 10.1016/j.immuni.2024.02.019.Dvorkin S, Cambier S, Volkman HE, et al. New frontiers in the cGAS�STING intracellular DNA-sensing pathway[ J]. Immunity, 2024, 57(4):
718-730. DOI: 10.1016/j.immuni.2024.02.019.
104、Gulen MF, Samson N, Keller A, et al. cGAS-STING drives ageing�related inflammation and neurodegeneration[ J]. Nature, 2023,
620(7973): 374-380. DOI: 10.1038/s41586-023-06373-1.Gulen MF, Samson N, Keller A, et al. cGAS-STING drives ageing�related inflammation and neurodegeneration[ J]. Nature, 2023,
620(7973): 374-380. DOI: 10.1038/s41586-023-06373-1.
105、Hopfner KP, Hornung V. Molecular mechanisms and cellular functions
of cGAS-STING signalling[ J]. Nat Rev Mol Cell Biol, 2020, 21(9):
501-521. DOI: 10.1038/s41580-020-0244-x.Hopfner KP, Hornung V. Molecular mechanisms and cellular functions
of cGAS-STING signalling[ J]. Nat Rev Mol Cell Biol, 2020, 21(9):
501-521. DOI: 10.1038/s41580-020-0244-x.
106、Te m p l e S. A d v a n c i ng c e l l t h e ra p y f o r n e u ro d e ge n e rat i v e
diseases[ J]. Cell Stem Cell,2023,30(5):512-529. DOI: 10.1016/
j.stem.2023.03.017.Te m p l e S. A d v a n c i ng c e l l t h e ra p y f o r n e u ro d e ge n e rat i v e
diseases[ J]. Cell Stem Cell,2023,30(5):512-529. DOI: 10.1016/
j.stem.2023.03.017.
107、Yu C, Roubeix C, Sennlaub F, et al. Microglia versus monocytes:
distinct roles in degenerative diseases of the retina[ J]. Trends Neurosci,
2020,43(6):433-449. DOI:10.1016/j.tins.2020.03.012Yu C, Roubeix C, Sennlaub F, et al. Microglia versus monocytes:
distinct roles in degenerative diseases of the retina[ J]. Trends Neurosci,
2020,43(6):433-449. DOI:10.1016/j.tins.2020.03.012
108、Fletcher EL. Contribution of microglia and monocytes to the
development and progression of age related macular degeneration[ J].
Ophthalmic Physiol Opt, 2020, 40(2): 128-139. DOI: 10.1111/
opo.12671.Fletcher EL. Contribution of microglia and monocytes to the
development and progression of age related macular degeneration[ J].
Ophthalmic Physiol Opt, 2020, 40(2): 128-139. DOI: 10.1111/
opo.12671.
109、Telegina DV, Kozhevnikova OS, Kolosova NG. Changes in retinal
glial cells with age and during development of age-related macular
degeneration[ J]. Biochemistry, 2018, 83(9): 1009-1017. DOI:
10.1134/S000629791809002X.Telegina DV, Kozhevnikova OS, Kolosova NG. Changes in retinal
glial cells with age and during development of age-related macular
degeneration[ J]. Biochemistry, 2018, 83(9): 1009-1017. DOI:
10.1134/S000629791809002X.
110、Karg MM, Moorefield M, Hoffmann E, et al. Microglia preserve visual
function loss in the aging retina by supporting retinal pigment epithelial
health[ J]. Immun Ageing, 2023, 20(1): 53. DOI: 10.1186/s12979-
023-00358-4.Karg MM, Moorefield M, Hoffmann E, et al. Microglia preserve visual
function loss in the aging retina by supporting retinal pigment epithelial
health[ J]. Immun Ageing, 2023, 20(1): 53. DOI: 10.1186/s12979-
023-00358-4.
111、Ma W, Zhao L, Fontainhas AM, et al. Microglia in the mouse retina
alter the structure and function of retinal pigmented epithelial cells:
a potential cellular interaction relevant to AMD[ J]. PLoS One, 2009,
4(11): e7945. DOI: 10.1371/journal.pone.0007945.Ma W, Zhao L, Fontainhas AM, et al. Microglia in the mouse retina
alter the structure and function of retinal pigmented epithelial cells:
a potential cellular interaction relevant to AMD[ J]. PLoS One, 2009,
4(11): e7945. DOI: 10.1371/journal.pone.0007945.
112、Ma W, Silverman SM, Zhao L, et al. Absence of TGFβ signaling
in retinal microglia induces retinal degeneration and exacerbates
choroidal neovascularization[ J]. Elife, 2019, 8: e42049. DOI: 10.7554/
eLife.42049.Ma W, Silverman SM, Zhao L, et al. Absence of TGFβ signaling
in retinal microglia induces retinal degeneration and exacerbates
choroidal neovascularization[ J]. Elife, 2019, 8: e42049. DOI: 10.7554/
eLife.42049.
113、Yu C, Lad EM, Mathew R, et al. Microglia at sites of atrophy restrict the
progression of retinal degeneration via galectin-3 and Trem2[ J]. J Exp
Med, 2024, 221(3): e20231011. DOI: 10.1084/jem.20231011.Yu C, Lad EM, Mathew R, et al. Microglia at sites of atrophy restrict the
progression of retinal degeneration via galectin-3 and Trem2[ J]. J Exp
Med, 2024, 221(3): e20231011. DOI: 10.1084/jem.20231011.
114、Shi Y, Zhang L, Teng J, et al. HMGB1 mediates microglia activation
via the TLR4/NF-κB pathway in coriaria lactone induced
epilepsy[ J]. Mol Med Rep, 2018, 17(4): 5125-5131. DOI: 10.3892/
mmr.2018.8485.Shi Y, Zhang L, Teng J, et al. HMGB1 mediates microglia activation
via the TLR4/NF-κB pathway in coriaria lactone induced
epilepsy[ J]. Mol Med Rep, 2018, 17(4): 5125-5131. DOI: 10.3892/
mmr.2018.8485.
115、Wang H , So ng X , L i M , e t a l . Th e ro l e o f T L R 4 / N F-κB
signaling pathway in activated microglia of rats with chronic high
intraocular pressure and vitro scratch injury-induced microglia[ J].
Int Immunopharmacol, 2020, 83: 106395. DOI: 10.1016/
j.intimp.2020.106395.Wang H , So ng X , L i M , e t a l . Th e ro l e o f T L R 4 / N F-κB
signaling pathway in activated microglia of rats with chronic high
intraocular pressure and vitro scratch injury-induced microglia[ J].
Int Immunopharmacol, 2020, 83: 106395. DOI: 10.1016/
j.intimp.2020.106395.
116、Angelova%20DM%2C%20Brown%20DR.%20Microglia%20and%20the%20aging%20brain%3A%20are%20senescent%20%0Amicroglia%20the%20key%20to%20neurodegeneration%3F%5B%20J%5D.%20J%20Neurochem%2C%202019%2C%20%0A151(6)%3A%20676-688.%20DOI%3A%2010.1111%2Fjnc.14860.Angelova%20DM%2C%20Brown%20DR.%20Microglia%20and%20the%20aging%20brain%3A%20are%20senescent%20%0Amicroglia%20the%20key%20to%20neurodegeneration%3F%5B%20J%5D.%20J%20Neurochem%2C%202019%2C%20%0A151(6)%3A%20676-688.%20DOI%3A%2010.1111%2Fjnc.14860.
117、Dissecting microglial aging and creating a model of aged microglia
in a non-aged brain[ J]. Nat Aging, 2023, 3(10): 1185-1186. DOI:
10.1038/s43587-023-00487-x.Dissecting microglial aging and creating a model of aged microglia
in a non-aged brain[ J]. Nat Aging, 2023, 3(10): 1185-1186. DOI:
10.1038/s43587-023-00487-x.
118、Ma W, Coon S, Zhao L, et al. A2E accumulation influences retinal
microglial activation and complement regulation[ J]. Neurobiol Aging,
2013, 34(3): 943-960. DOI: 10.1016/j.neurobiolaging.2012.06.010.Ma W, Coon S, Zhao L, et al. A2E accumulation influences retinal
microglial activation and complement regulation[ J]. Neurobiol Aging,
2013, 34(3): 943-960. DOI: 10.1016/j.neurobiolaging.2012.06.010.
119、Wong W. The aging phenotype of microglia in the retina and its
relationship to AMD[ J]. Acta Ophthalmol, 2014, 92(s253). DOI:
10.1111/j.1755-3768.2014.1752.xWong W. The aging phenotype of microglia in the retina and its
relationship to AMD[ J]. Acta Ophthalmol, 2014, 92(s253). DOI:
10.1111/j.1755-3768.2014.1752.x
120、Daley R , Maddipatla V, Ghosh S, et al. Aberrant Akt2 signaling
in the RPE may contribute to retinal fibrosis process in diabetic
retinopathy[ J]. Cell Death Discov, 2023, 9(1): 243. DOI: 10.1038/
s41420-023-01545-4.Daley R , Maddipatla V, Ghosh S, et al. Aberrant Akt2 signaling
in the RPE may contribute to retinal fibrosis process in diabetic
retinopathy[ J]. Cell Death Discov, 2023, 9(1): 243. DOI: 10.1038/
s41420-023-01545-4.
121、Song Y, Liao Y, Liu T, et al. Microglial repopulation restricts ocular
inflammation and choroidal neovascularization in mice[ J]. Front
Immunol, 2024, 15: 1366841. DOI: 10.3389/fimmu.2024.1366841.Song Y, Liao Y, Liu T, et al. Microglial repopulation restricts ocular
inflammation and choroidal neovascularization in mice[ J]. Front
Immunol, 2024, 15: 1366841. DOI: 10.3389/fimmu.2024.1366841.
122、Huang H, Parlier R, Shen JK, et al. VEGF receptor blockade markedly
reduces retinal microglia/macrophage infiltration into laser-induced
CNV[ J]. PLoS One, 2013, 8(8): e71808. DOI: 10.1371/journal.
pone.0071808.Huang H, Parlier R, Shen JK, et al. VEGF receptor blockade markedly
reduces retinal microglia/macrophage infiltration into laser-induced
CNV[ J]. PLoS One, 2013, 8(8): e71808. DOI: 10.1371/journal.
pone.0071808.
123、Trimm E, Red-Horse K. Vascular endothelial cell development and
diversity[ J]. Nat Rev Cardiol, 2023, 20(3): 197-210. DOI: 10.1038/
s41569-022-00770-1.Trimm E, Red-Horse K. Vascular endothelial cell development and
diversity[ J]. Nat Rev Cardiol, 2023, 20(3): 197-210. DOI: 10.1038/
s41569-022-00770-1.
124、Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. Role of the retinal
vascular endothelial cell in ocular disease[ J]. Prog Retin Eye Res, 2013,
32: 102-180. DOI: 10.1016/j.preteyeres.2012.08.004.Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. Role of the retinal
vascular endothelial cell in ocular disease[ J]. Prog Retin Eye Res, 2013,
32: 102-180. DOI: 10.1016/j.preteyeres.2012.08.004.
125、Arthur E, Alber J, Thompson LI, et al. OCTA reveals remodeling of
the peripheral capillary free zones in normal aging[ J]. Sci Rep, 2021,
11(1): 15593. DOI: 10.1038/s41598-021-95230-0.Arthur E, Alber J, Thompson LI, et al. OCTA reveals remodeling of
the peripheral capillary free zones in normal aging[ J]. Sci Rep, 2021,
11(1): 15593. DOI: 10.1038/s41598-021-95230-0.
126、Bertelli PM, Pedrini E, Hughes D, et al. Long term high glucose
exposure induces premature senescence in retinal endothelial cells[ J].
Front Physiol, 2022, 13: 929118. DOI: 10.3389/fphys.2022.929118.Bertelli PM, Pedrini E, Hughes D, et al. Long term high glucose
exposure induces premature senescence in retinal endothelial cells[ J].
Front Physiol, 2022, 13: 929118. DOI: 10.3389/fphys.2022.929118.
127、Hurtley SM. Remodeling senescent blood vessels[ J]. Science, 2020,
369(6506): 930-932. DOI: 10.1126/science.369.6506.930-k.Hurtley SM. Remodeling senescent blood vessels[ J]. Science, 2020,
369(6506): 930-932. DOI: 10.1126/science.369.6506.930-k.
128、Yin Y, Zhou Z, Liu W, et al. Vascular endothelial cells senescence is
associated with NOD-like receptor family pyrin domain-containing 3
(NLRP3) inflammasome activation via reactive oxygen species (ROS)/
thioredoxin-interacting protein (TXNIP) pathway[ J]. Int J Biochem
Cell Biol, 2017, 84: 22-34. DOI: 10.1016/j.biocel.2017.01.001.Yin Y, Zhou Z, Liu W, et al. Vascular endothelial cells senescence is
associated with NOD-like receptor family pyrin domain-containing 3
(NLRP3) inflammasome activation via reactive oxygen species (ROS)/
thioredoxin-interacting protein (TXNIP) pathway[ J]. Int J Biochem
Cell Biol, 2017, 84: 22-34. DOI: 10.1016/j.biocel.2017.01.001.
129、Binet F, Cagnone G, Crespo-Garcia S, et al. Neutrophil extracellular
traps target senescent vasculature for tissue remodeling in
retinopathy[ J]. Science, 2020, 369(6506): eaay5356. DOI: 10.1126/
science.aay5356.Binet F, Cagnone G, Crespo-Garcia S, et al. Neutrophil extracellular
traps target senescent vasculature for tissue remodeling in
retinopathy[ J]. Science, 2020, 369(6506): eaay5356. DOI: 10.1126/
science.aay5356.
130、Smith TL, Oubaha M, Cagnone G, et al. eNOS controls angiogenic
sprouting and retinal neovascularization through the regulation of
endothelial cell polarity[ J]. Cell Mol Life Sci, 2021, 79(1): 37. DOI:
10.1007/s00018-021-04042-y.Smith TL, Oubaha M, Cagnone G, et al. eNOS controls angiogenic
sprouting and retinal neovascularization through the regulation of
endothelial cell polarity[ J]. Cell Mol Life Sci, 2021, 79(1): 37. DOI:
10.1007/s00018-021-04042-y.