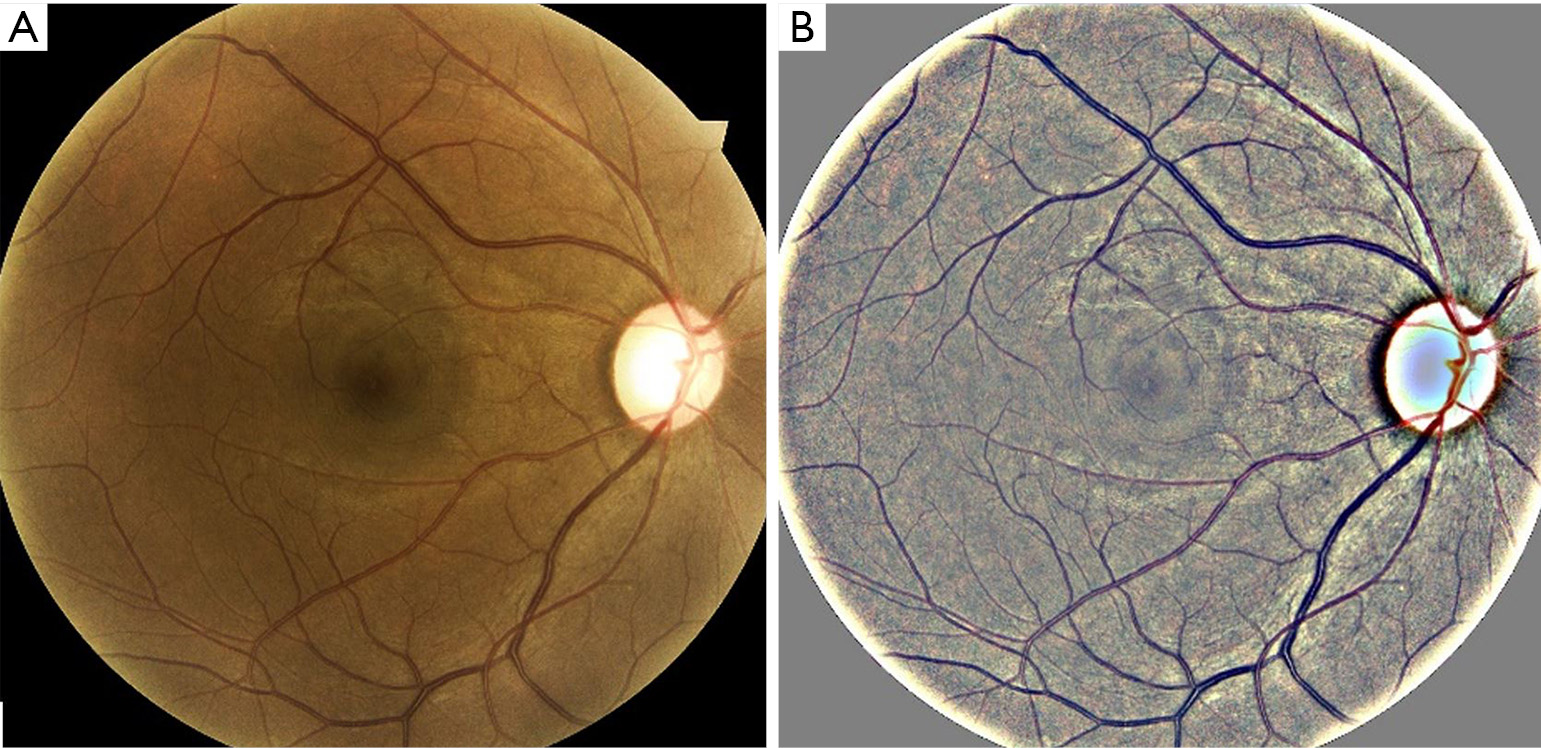
Age-related macular degeneration (AMD) and diabetic retinopathy (DR) (shown in Figure 1) are some of the most common blinding diseases affecting millions of people worldwide (1-4). AMD is the leading cause of vision loss in those over 50 years of age in the developed world (5-7). The number of people with AMD is expected to increase 1.5-fold over ten years due to our aging population, hypertension, and other causes (8). It is often too late to mitigate the complications by the time a person visits an ophthalmologist, as the treatments cannot regenerate vision (9,10). Further, such treatments are expensive, typically costing up to $65,000 for one eye, depending on the drug used, for a 2-year course of treatment (11). While the total (direct and indirect in the USA) cost of AMD is $225 billion per year (5) and is expected to increase, the indirect cost is even greater due to injury, depression, and social dependency resulting from blindness (12).

Diabetic Retinopathy (4) is one of the leading causes of blindness, regardless of age, in the developed world. In the US, the number of patients suffering from DR is expected to reach 6 million by 2020 and 11.3 million by 2030 (13). Early detection of the disease is key to its effective treatment and subsequent reduction of associated economic burdens. The total annual economic burden of eye diseases in the US is about $139B (13).
Our literature review found a number of screening models (14-16) for detecting AMD automatically but without validation from external data or derived from very few test images. Among recent advances in deep learning (DL) is a method proposed by Liu et al. (17), which utilized multiple instances of learning to produce a model built with under 5,000 fundus images and an area under the curve (AUC) of 0.79. Several studies focused on automated screening of DR and have achieved varying performance (18,19). Gulshan et al. (20) applied deep learning for DR detection and concluded that further research was needed to bring it to a clinical setting. Abràmoff et al. (21) proposed a similar algorithm with 87% sensitivity and 90% specificity. Ting et al. (22) proposed and validated a deep learning system built with data from multiethnic populations and compared it with professional human graders. Ting’s results showed a sensitivity of 90.5%, and a specificity of 91.6% for detecting referable DR. Gargeya et al. (23) proposed a similar deep learning-based model with an AUC of 0.94, a sensitivity of 93%, and a specificity of 87% on a public dataset. However, these models, all built on retrospective datasets, still need validation in real-world primary care clinical settings and a prospective study, which is the paper’s motivation.
Telemedicine platforms using cloud-based applications have helped increase the rate of screening for eye diseases, with one study reporting an increase of diabetes-related retinal exams from 37% to 87% (24). Studies have concluded that cloud-based DR screening can identify up to 25% more cases in the diabetic population (25). It has also been shown that telemedicine screening of diseases reduces costs significantly (26). Therefore, we have combined the AMD and DR screening tools on a secure HIPAA compliant telemedicine platform (27,28) to screen patients for the two eye diseases without additional imaging or visits.
This study demonstrates the validity and suitability of the screening system for AMD and DR on prospective data in clinical settings. We present this study in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/aes-20-114).
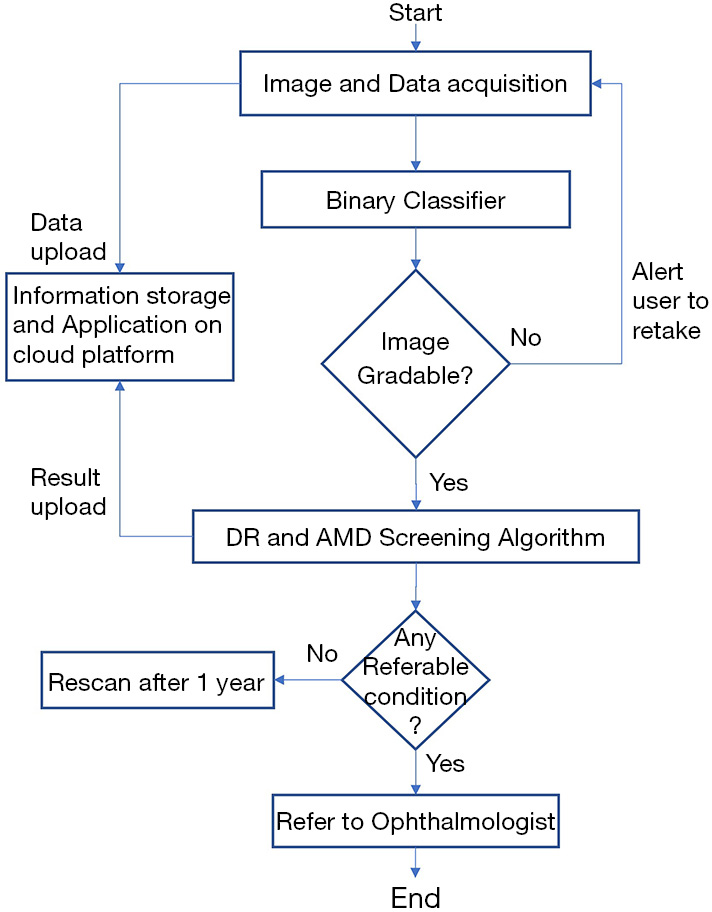
A patient’s retinal color fundus images are taken in a clinical setting then uploaded to the iHealthScreen developed cloud-based telemedicine platform by the healthcare worker. If the image is deemed ungradable by the system, it prompts the user to upload a new image. Once the image is accepted, the AI-powered automated AMD and DR screening algorithms perform evaluations and send back reports about the referability with respect to the two diseases individually. Based on this report, the patient would be referred to an ophthalmologist if needed. The method is explained in detail in the following paragraphs. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Mount Sinai (# IRB-18-00778) and informed consent was taken from all the patients.
The screening system, built using recent advances in machine learning and artificial intelligence, accepts retinal color fundus images, which can be taken from a wide variety of cameras and imaging conditions. The images used in testing the systems were captured without pharmacological dilation with a Topcon TRC NW6 non-mydriatic fundus camera (Topcon Corp., Tokyo, Japan) with a 45-degree field of view, the DRS camera from CenterVue Inc. and the Eidon camera (Eidon, CenterVue Inc., Fremont, CA) with a 45-degree field of view. Images were preprocessed before training the models to enhance the robustness of the systems. A local color averaging technique was used to eliminate lighting gradients in the fundus image. An example of such a technique is shown in Figure 2. The screening systems referenced later in this paper have performed well under different imaging conditions.
iHealthScreen Inc. developed an artificial intelligence (AI) based telemedicine platform (28) that integrates the server-side programs for image analysis and deep-learning (29) modules intended for screening system, with local remote computer or mobile devices for collecting patient data and images. The images are first checked for gradability automatically by AI developed in-house that achieved over 99% accuracy on 3,000 fundus images. Once the check is passed, the remote devices in primary care will upload images and data to the server for automatic analysis, as shown in Figure 3. The telemedicine platform is compatible with both web and mobile platforms. It sends a report to the remote devices with an individual’s screening results of the two eye diseases and further recommendations to visit an ophthalmologist. The entire process from data entry to image analysis report is determined to take only a few minutes, depending on the user’s experience in handling the equipment, saving time for both the doctor and the patient. The client-side app will call the clinical decision support system to access the data, perform automated screening, and decide if a referral to an ophthalmologist is necessary.
Deep learning (29) is a popular technique that has been recently used for eye disease screening. Deep learning is a class of machine learning techniques that allows systems to learn features directly from the data without having to specify any rules or conditions about predictive parameters if there is sufficiently large labeled data input. Deep learning has also been applied in medical applications to detect various diseases such as macular degeneration (30), melanoma (31), and others. Our two models use recent advances in deep learning and artificial intelligence to produce highly accurate classifiers.
The AMD screening system (32-34) was developed, tested, and validated by iHealthScreen Inc. for identifying referable AMD patients. An ensemble of deep learning screening methods was trained and validated on 116,875 color fundus photos from 4,139 participants in the Age-Related Eye Diseases study (35) to classify them as normal (healthy), early, intermediate, or advanced AMD based on the presence and extent of retinal abnormalities. This study evaluated the system’s performance as a binary classifier—referable (intermediate/late) and non-referable (normal/early). For the identification of referable AMD over non-referable, the system achieved 99.2% accuracy, with a sensitivity of 98.9% and a specificity of 99.5%.
The DR screening model (36,37) was developed by iHealthScreen Inc. using deep learning techniques and tested using 88702 images from the Kaggle dataset (38) and externally validated using 1,748 high-resolution fundus images from the Messidor-2 (39) dataset. The images were uploaded in the cloud-based software for testing the automated DR screening platform. The system accepts a fundus image and automatically grades it on a five-point scale—normal, mild, moderate, severe non-proliferative with diabetic macular edema and proliferative DR. An image is considered as referable DR if the grade is moderate or worse. Otherwise, it is considered as non-referable. The automated referable and non-referable DR system evaluation is compared against the expert ophthalmologists’ evaluation. The screening system, used on the Kaggle dataset, achieved a sensitivity of 99.2%, a specificity of 97.6%, and an AUC of 0.99 when identifying referable DR. The system was also externally validated in Messidor-2, where it achieved a sensitivity of 97.6%, a specificity of 99.5%, and an AUC of 0.99.
For AMD and DR, 340 non-dilated subjects aged over 50 years had retinal color imaging of both eyes performed randomly at New York Eye and Ear faculty retina practices with an FDA approved color fundus camera (Eidon, CenterVue Inc., Fremont, CA, USA) between October 2019 and April 2020, and 152 diabetic patients at New York Eye and Ear faculty retina practices, ophthalmic and primary care clinics, yielding 984 images. After exclusion of 308 images with other confounding conditions like myopia and vascular occlusion, and those of poor quality, a total of 676 images were selected and evaluated for AMD and DR. It should be noted that, while in practice, patient referral is based on the worse eye, the models are trained and evaluated on per eye image basis. All images were uploaded to the telemedicine platform and analyzed by the appropriate screening systems.
Three expert graders also classified patients’ eyes as referable AMD (intermediate or late AMD) or non-referable (healthy macula or early AMD). Separately, they were also classified as referable DR and non-referable DR. After adjudication of disagreement in the grading to consensus, 172 were referable, and 504 eyes were non-referable AMD. Similarly, 33 were referable, and 643 were non-referable DR.
With a referable case as “positive” and non-referable case as “negative”, clinically relevant measures such as sensitivity, specificity, accuracy, and kappa scores were calculated, with 95% confidence intervals. For the two diseases, 2×2 tables were generated to characterize the algorithm’s sensitivity and specificity with respect to the reference standard. The reference standard was defined as the majority decision of the experts’ grading. The 95% confidence intervals for the sensitivity and specificity of the algorithm were calculated to be exact Clopper-Pearson intervals (40), which corresponded with individual coverage probabilities of sqrt (0.95) ~?0.975.
Intergrader reliability was measured among the human graders using kappa scores. The graders’ disagreements were adjudicated by taking the majority grade among the graders (two of three grader agreement). The human grader disagreement is an important measure to compare system performance with that of humans. The grades thus obtained are used to measure the performance of our DR and AMD screening system.
To assess disagreement among the three graders, we chose the AMD dataset and kappa score measures. The majority grading, two of three graders agree, was compared to each of the graders, and kappa scores were calculated. Against majority grading, grader 1 had a kappa score of 0.71, while grader 2 scored 0.95, and grader 3 scored 0.88 (see Table 1).
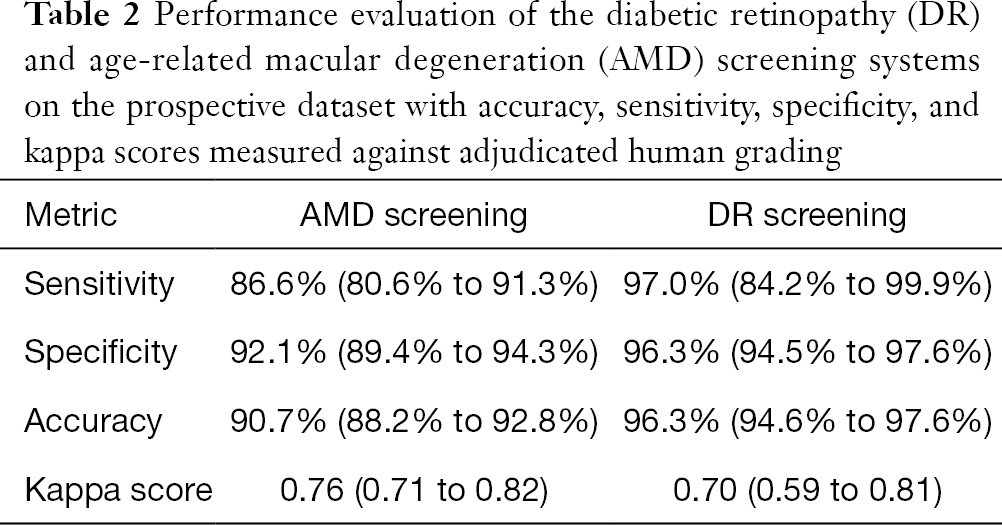
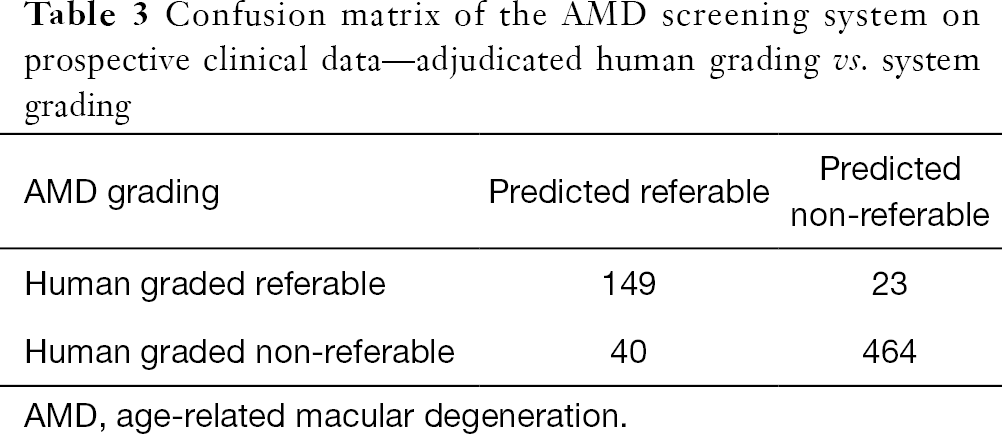
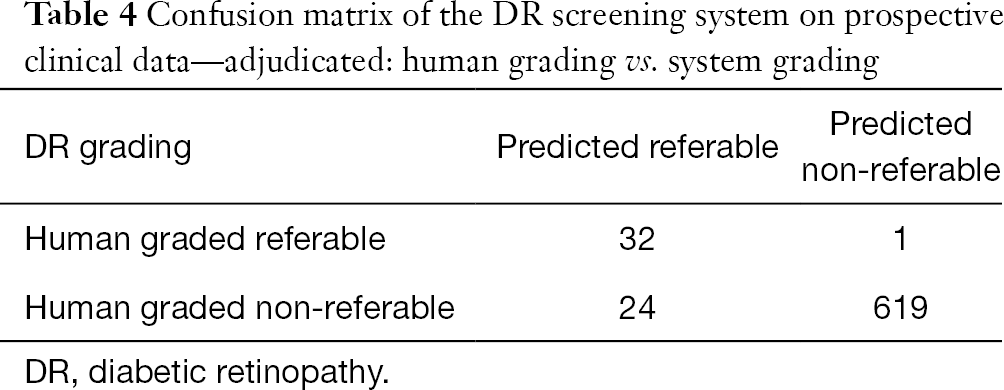
Against the majority agreement grading, the AMD screening system (see Tables 2 and 3) achieved a sensitivity of 86.6% (80.6% to 91.3%), a specificity of 92.1% (89.4% to 94.3%), an accuracy of 90.7% (88.2% to 92.8%), and a kappa score of 0.76 (0.71 to 0.82) on the prospective dataset. The DR screening system achieved (see Tables 2 and 4) a sensitivity of 97.0% (84.2% to 99.9%), a specificity of 96.3% (94.5% to 97.6%), an accuracy of 96.3% (94.6% to 97.6%), and a kappa score of 0.70 (0.59 to 0.81).
In this study, we have used two prospective datasets and demonstrated the suitability of an AI-based automated combined screening platform for AMD and DR in clinical settings. The screening platform can fill an unmet need for screening individuals with AMD and DR through a regular primary care doctor’s visit. A study from the National Eye Institute showed that half of the patients do not obtain eye examinations recommended by their general physicians. Optometrists and ophthalmologists who screen for DR and AMD are often limited geographically and have limited time. Specialist visits are also time-consuming. In these instances, automated screening tools within the primary care setting will help mitigate these issues and provide better care for patients with these eye diseases.
We have developed and validated the telemedicine-ready, AI-based, and fully automated screening tools that can be used for screening these diseases with just one image per eye in one visit. Evaluation of the systems in primary care clinics validated the high accuracy of these screening tools, comparable to that of human graders. We note that we used different retinal color fundus cameras (Topcon TRC NW6 non-mydriatic cameras) for developing the model than those used to obtain the prospective data (DRS and Eidon cameras from CenterVue Inc.). Thus, our results are already robust to various types of cameras in the development and test phases and, with larger image sets from each camera, can surely produce even better results.
These AI-based screening tools should be tested prospectively in more diverse clinical settings with cost and time analyses for establishing reliability, consistency, and quantifying individual cost and time benefits. While two eye diseases can be screened simultaneously, the system is limited to only DR and AMD. Other eye diseases can potentially be screened using the same fundus image, such as glaucoma and hypertensive retinopathy. A non-referable grade in either AMD or DR speaks only to its referability for the said diseases. That means other non-AMD and non-DR related pathologies, which the ophthalmologists would have picked up while reviewing the fundus images, can be missed by the more targeted automated grading systems. Making the automated systems more versatile in detecting other pathologies is warranted.
As evident from the disagreements in specialist human graders and their comparisons with the screening systems’ kappa scores, it can be concluded that the automated tools perform as well as the human graders, if not better. The performance of the tools is confirmed in the context of real-world image acquisition and analysis. The physical system and the telemedicine software were tested for usability, convenience, and security. The screening systems were deployed on HIPAA-compliant telemedicine platforms and built for minimum interaction with the interface. By using such a secure, fast, reliable, and low-cost system, millions of eyes can potentially be saved from preventable vision-loss, with significant healthcare savings.