Abstract: Pathologic myopia is the major cause of the loss of the best-corrected visual acuity (BCVA) worldwide, especially in East Asian countries. The loss of BCVA is caused by the development of myopic macula patchy, myopic traction macula patchy, and myopic optic neuropathy (or glaucoma). The development of such vision-threatening complications is caused by eye deformity, characterized by a formation of posterior staphyloma. The recent advance in ocular imaging has greatly facilitated the clarification of pathologies and pathogenesis of pathological myopia and myopia-related complications. These technologies include ultra-wide field fundus imaging, swept-source optical coherence tomography, and 3D MRI. In addition, the new treatments such as anti-VEGF therapies for myopic choroid all neovascularization have improved the outcome of the patients. Swept-source OCT showed that some of the lesions of myopic maculopathy were not simply chorioretinal atrophy but were Bruch’s membrane holes. Features of myopic traction maculopathy have been analyzed extensively by using OCT. The understanding the pathophysiology of complications of pathologic myopia is considered useful for better management of this blinding eye disease.
Complications from pathologic myopia (PM) are a major cause of the loss of the best-corrected visual acuity (BCVA), especially in East Asia (1-6). The loss of BCVA in addition to the decrease of uncorrected visual acuity due to its specific complications is a major feature of PM. Complications of PM develop mainly in the macula and in the optic nerve area. The deformity of the globe including posterior staphyloma may facilitate the development of these pathologies.
The definition of “PM” has not been standardized among studies, and other terms like “high myopia (HM)” have been used similarly. However, “HM” means a high degree of myopia and does not always mean the presence of complications causing the BCVA decrease.
In most early epidemiologic studies, the refractive error [?5.0 diopters (D), ?6.0 D, ?8.0 D], the axial length (>26.0 mm, >26.5 mm), or a combination of both have been used to define “PM”. However, a definition based on refractive error or increased axial length is considered to just show a “high degree of myopia (= HM).” In addition, there is no clear scientific basis for why these cutoff values were chosen.
Excessive elongation of the globe and posterior staphyloma are believed to be important factors in the development of posterior fundus lesions in PM (7-11). However, the refractive error or axial length alone often does not adequately reflect the ‘PM’. Posterior staphyloma, which is a hallmark lesion of PM, can occur also in non-highly myopic eyes (12,13). Recently an international panel of researchers in myopia reviewed previous published studies and classifications and proposed a simplified, uniform classification system for PM for use in future studies (14). In this META-PM (meta analyses of PM) study classification, PM was defined as the eyes having chorioretinal atrophy equal to or more severe than diffuse atrophy or as the eyes having posterior staphyloma (15,16).
Curtin and Karlin first proposed a definition of myopic maculopathy that included the features of chorioretinal atrophy, central pigment spot, lacquer cracks, posterior staphyloma and optic disc changes in 1970 (12). Later, Tokoro (17) updated the classification of myopic macular lesions into four categories: (I) tessellated fundus; (II) diffuse chorioretinal atrophy; (III) patchy chorioretinal atrophy; and (IV) macular hemorrhage. Subsequently, Avila et al. (18) developed a classification which included myopic retinopathy according to severity, from M0-normal-appearing posterior pole; M1-choroidal pallor and tessellation; M2-M1 changes with posterior staphyloma; M3-M2 changes with lacquer cracks; M4-M3 changes with focal areas of deep choroidal atrophy; to the most severe grade M5-M4 changes with large geographic areas of deep chorioretinal atrophy and bare sclera.
Recently an international panel of researchers in myopia reviewed previous published studies and classifications and proposed a simplified, uniform classification system for PM for use in future studies (14). In this simplified system (META-PM classification; Table 1), myopic maculopathy lesions are categorized into 5 categories from “no myopic retinal lesions” (Category 0), “tessellated fundus only” (Category 1), “diffuse chorioretinal atrophy” (Category 2; Figure 1A), “patchy chorioretinal atrophy” (category 3; Figure 1B), to “macular atrophy” (Category 4). Three additional features were added to these categories and were included as “plus signs”: (I) lacquer cracks (Figure 1C); (II) myopic CNV (Figure 1D); and (III) Fuchs spot. The reason for separately defining these “plus signs” is that these 3 lesions have been shown to be strongly associated with central vision loss, but they do not fit into any particular category and may develop from, or coexist, in eyes with any of the myopic maculopathy categories described above. Based on this new classification, PM is defined as myopic maculopathy category 2 or above, or presence of “plus” sign, or the presence of posterior staphyloma (15,19).
| META-PM classification | Myopic retinal changes | Fundus appearance |
|---|---|---|
| Category 0 | No myopic retinal changes | – |
| Category 1 | Tessellated fundus | Well-defined choroidal vessels can be observed clearly around the fovea and arcade vessels |
| Category 2 | Diffuse chorioretinal atrophy | The posterior pole appears yellowish white, extent of which is variable |
| Category 3 | Patchy chorioretinal atrophy | Well-defined, grayish white lesions, size variable between 1 and several choroidal lobules |
| Category 4 | Macular atrophy | Well-defined, round chorioretinal atrophic lesion that is grayish white or whitish around a regressed fibrovascular membrane that enlarges with time. Generally macular atrophy is centered on the central fovea and has a round shape |
| Lacquer cracks | Yellowish thick linear pattern | |
| Choroidal neovascularization | Active CNV should be accompanied by exudative activity or hemorrhage. Serous retinal detachments can be present | |
| Fuchs spot | Pigmented spot representing the dry fibrovascular scar of myopic CNV |
CNV, choroidal neovascularization.
Currently, the updated modification of the META-PM classification is going on based on our recent longer follow-up study (>10 years). A longer follow-up data showed that the progression from C3 to C4 is uncommon, and most of the C4 is myopic CNV-related. In addition, Fuchs spot is pigmented scar of myopic CNV. Not all of scar phase of myopic CNV shows an increased pigmentation. Thus, it might be better not to separately identify Fuchs spot and better to have active phase and scar phase together under the category of myopic CNV. Finally, the lesions from C2 through C4 are specific to PM, whereas C1 is seen even in mild myopia and C0 is just normal fundus. Thus, new classification is expected to include only the lesions specific to PM (thus, C2, C3, C4, lacquer cracks, myopic CNV) and it is better to call “PM maculopathy”. The updated classification based on a longer follow-up study is expected to be published soon.
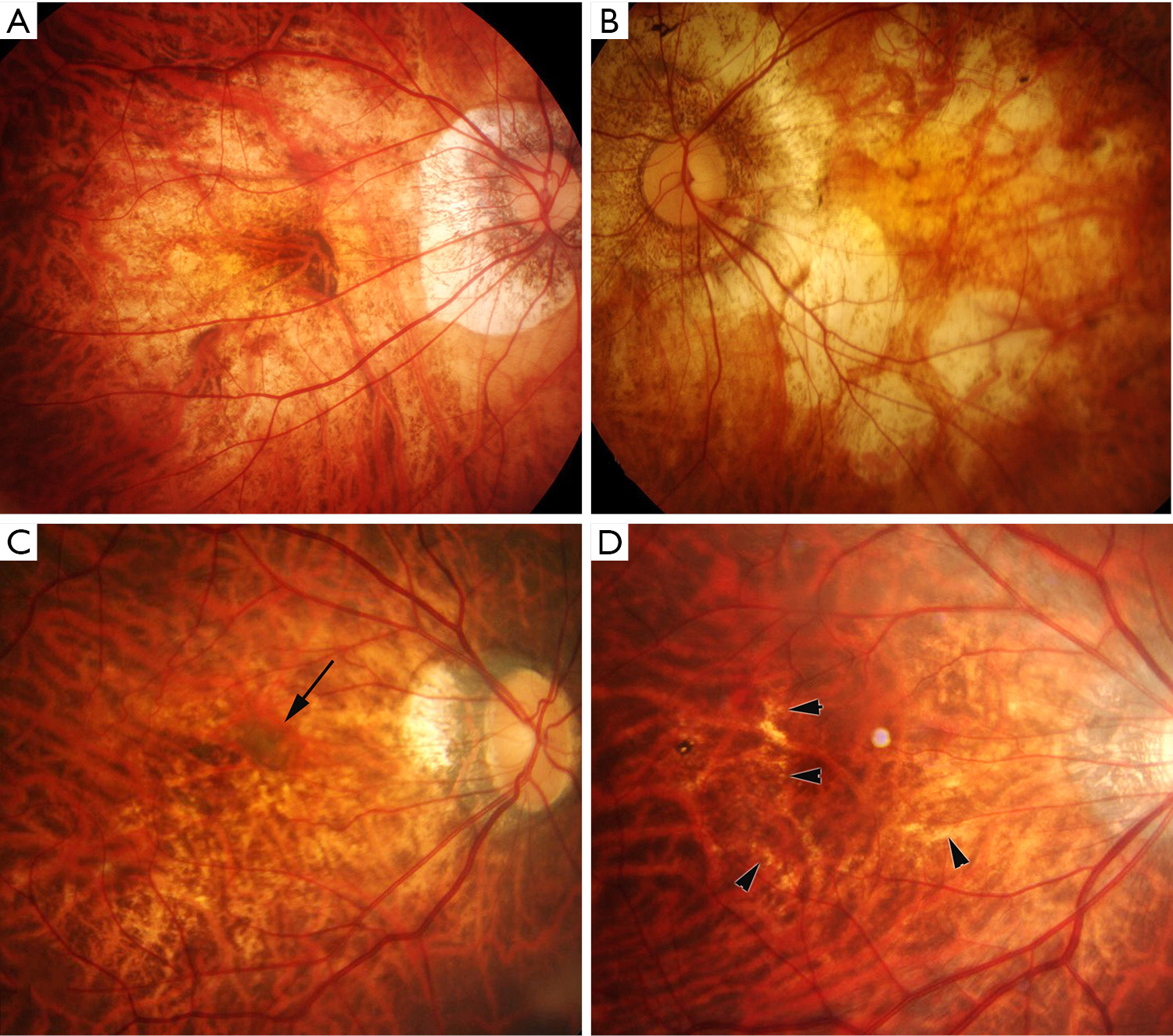
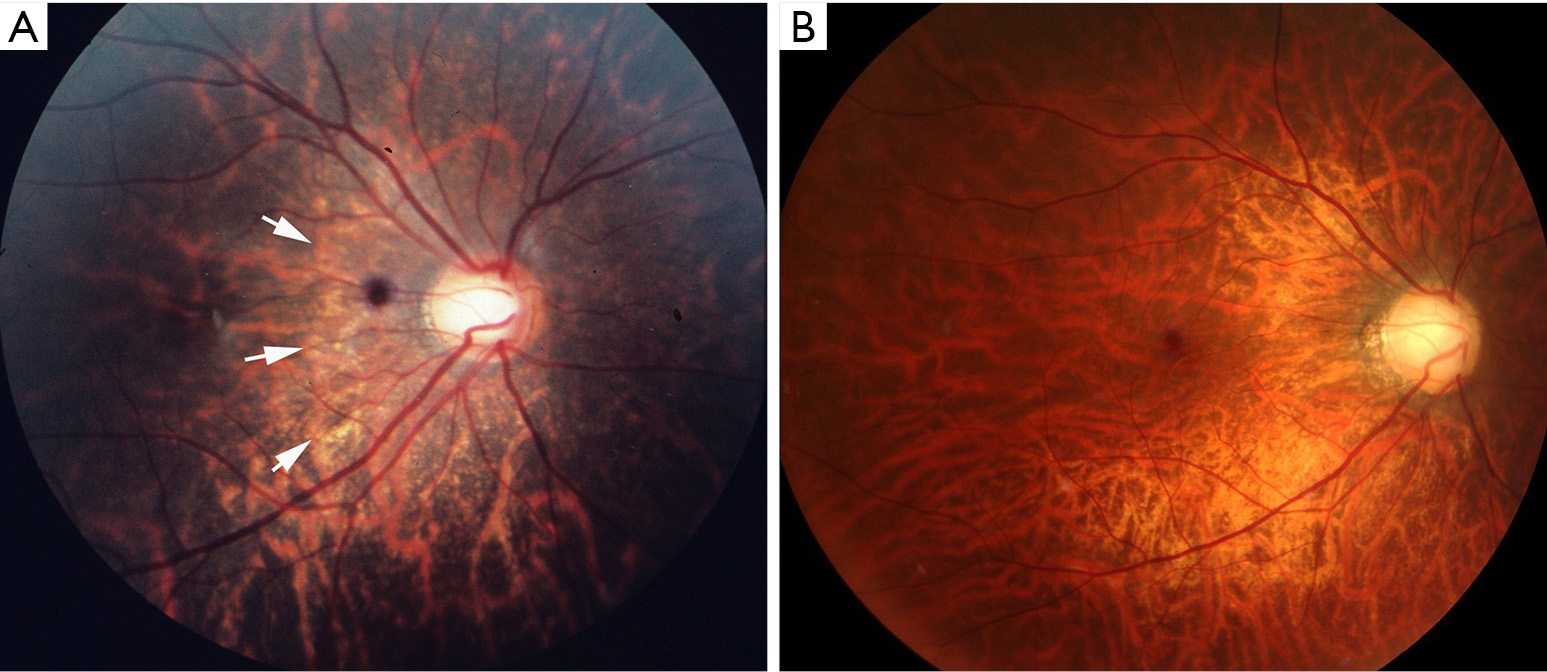
Diffuse chorioretinal atrophy is observed as ill-defined yellowish lesion in the posterior fundus of highly myopic eyes (Figure 1). This lesion begins to appear around the optic disc (peripapillary diffuse chorioretinal atrophy; PDCA) and enlarges with age and finally covers the entire area within the staphyloma (Figure 2).
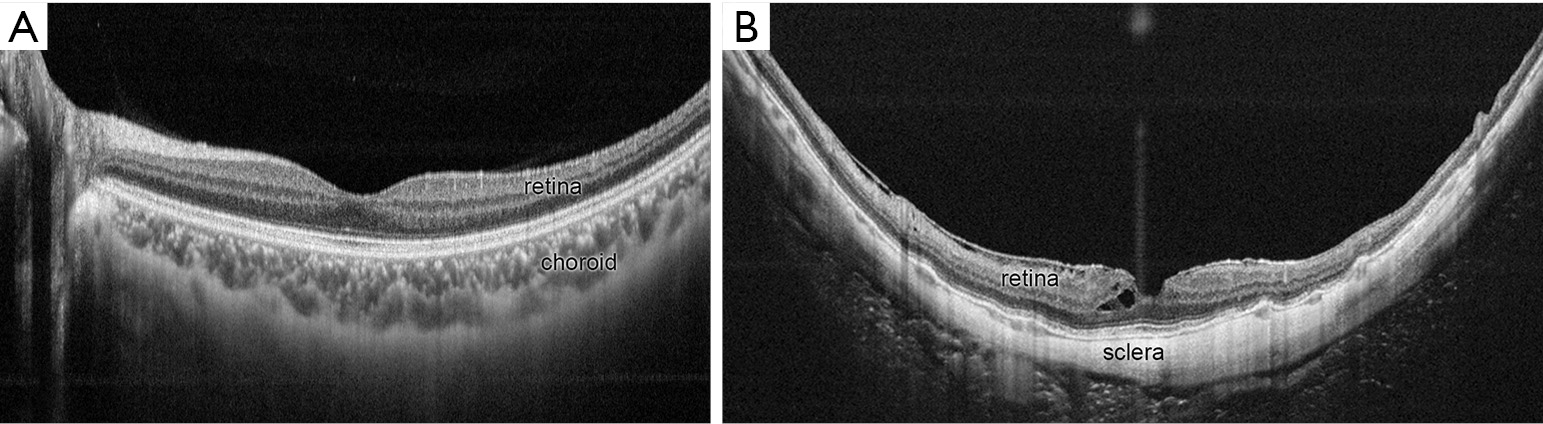
The main feature of diffuse atrophy is a marked thinning (almost absence) of choroid. Optical coherence tomography (OCT) shows a marked thinning of the choroid in the area of diffuse atrophy (Figure 3). In most of the cases, the choroid is almost absent except sporadically-present large choroidal vessels. A presence of outer retina and RPE even in the area where most of the choroid is gone might explain a relatively preserved vision in eyes with diffuse atrophy. Okisaka reported that the choroidal changes in PM began from the obliteration of precapillary arterioles or postcapillary venules, then followed by an occlusion of choriocapillaris. Finally, large choroidal vessels are also obliterated and the choroid seems to be absent. In parallel to the vascular changes in the choroid, choroidal melanocytes disappear as well. Although a choroid becomes thinned in eyes with tessellated fundus, the degree of choroidal thinning is much more serious in eyes with diffuse atrophy. And such disproportionate thinning of choroid compared to the surrounding tissue (RPE, outer retina, and sclera) might be a key phenomenon in diffuse atrophy.
Recently, Yokoi et al. (20) reported that in 83% of the adults with PM had had PDCA in their childhood in a long-term follow-up study >20 years since childhood. OCT showed that PDCA was sudden and focal loss of peripapillary choroid (21).
Patchy chorioretinal atrophy is observed as a grayish-white, well-defined atrophy (Figure 1) (17). Due to an absence of RPE and most of the choroid, the sclera can be observed through transparent retinal tissue, which is considered to show white color. Large choroidal vessels and intrascleral vessels seem to course within the area of patchy atrophy. In some cases, retrobulbar blood vessels are observed through the patchy atrophy with moving according to the gaze shift. Fluorescein angiogram (FA) as well as indocyanine green angiogram (ICGA) shows a choroidal filling defect in the area of patchy atrophy, suggesting that this lesion is a complete closure of choriocapillaris (17).
By using a recent imaging technology; swept-source OCT, Ohno-Matsui et al. (22) recently reported that patchy atrophy was not simply a chorioretinal atrophy but it was a hole of BM (Figure 4). In the area of patchy atrophy without BM, most of the thickness of choroid, RPE, and outer retina are lost and the inner retina directly sits on the sclera. This is contrary to the fact that the RPE and outer retina are preserved in most of the eyes with diffuse atrophy, although it is not certain if the remaining photoreceptors and RPE function normally in those eyes.
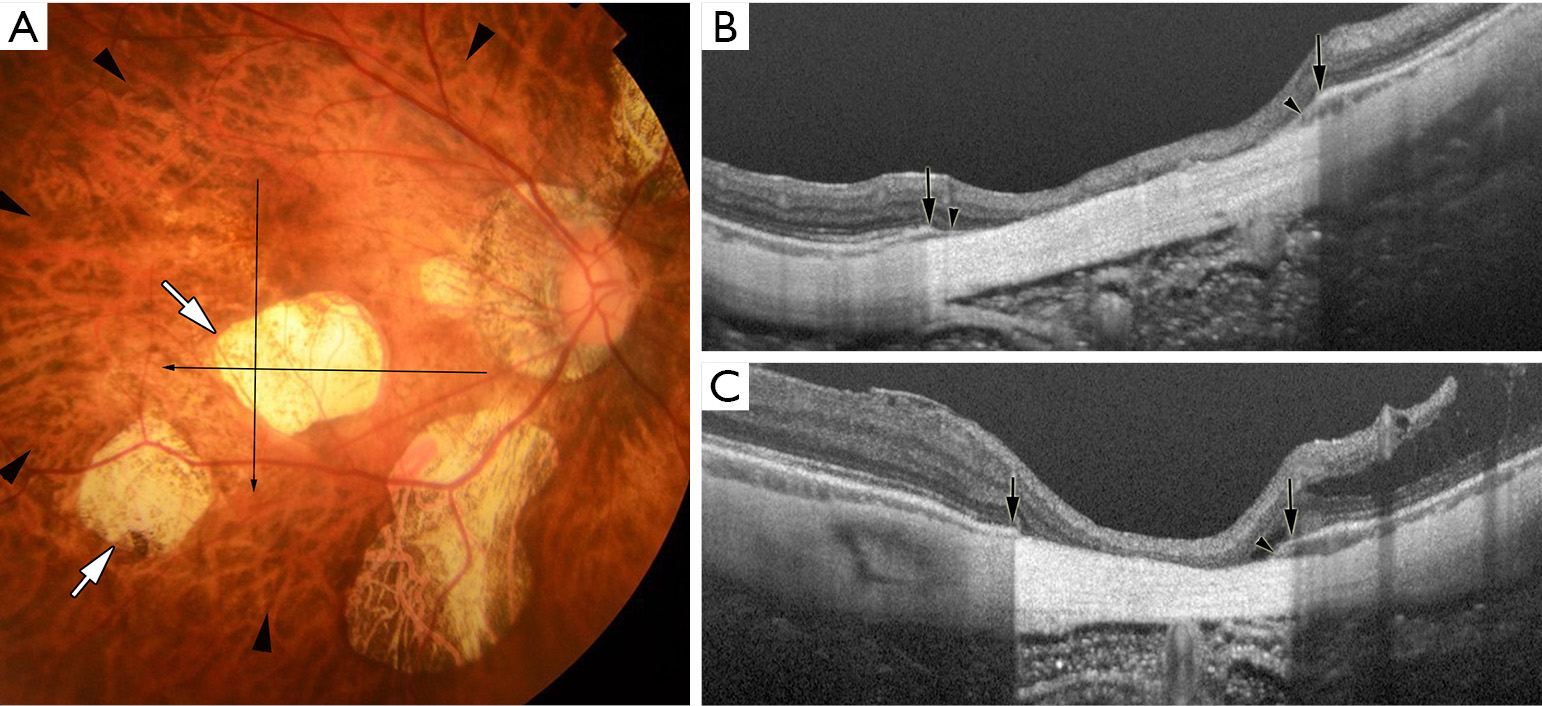
Patchy atrophy is subclassified into three types; patchy atrophy which develops from lacquer cracks; P(Lc), patchy atrophy which develops within the area of an advanced diffuse chorioretinal atrophy; P(D), and patchy atrophy which can be seen along the border of the posterior staphyloma; P(St) (23). P(Lc) is considered an enlarged break of BM at the site of Lc. P(St) is considered a mechanical break of BM at the staphyloma edge. P(D) is newly-developed BM holes in the area of extremely thin choroid.
Jonas et al. (24) recently reported that macular BM defects were found in 30.8% of highly myopic eyes whose axial length was ≥26.5 mm histologically. A lack of BM defects might be related to the development of macular ICC. Due to a lack of tensile BM in addition to a lack of choroid on the thin sclera, the area of patchy atrophy is very fragile against the inner pressure load. The sclera can be bowed posteriorly in the area of the patchy atrophy, which resembles the intrachoroidal cavitation that develops adjacent to a myopic conus in highly myopic eyes (peripapillary intrachoroidal cavitations; peripapillary ICC).
Ito-Ohara et al. (25) examined the direction of enlargement of patchy atrophy, and found that the patchy atrophy in marginal lesions of a staphyloma enlarged toward the macula, and the patchy atrophy in the macula enlarged in all directions (25). However, it is uncommon for extrafoveal patchy atrophy later involves the central fovea. This means that it is rare for patchy atrophy causes the central vision loss although this lesion causes a paracentral absolute scotoma due to a loss of photoreceptors within the atrophic area.
Lacquer cracks are fine, irregular, yellow lines, often branching and crisscrossing, seen in the posterior fundus of highly myopic eyes (Figure 1). Curtin and Karlin (12) reported that lacquer cracks were found in 4.3% of highly myopic eyes. Histologically, the lacquer cracks represent healed mechanical fissures in the RPE-BM-choriocapillaris complex (26).
Lacquer cracks can develop at a relatively early age in highly myopic patients (e.g., in the 30’s). The greatest incidence of lacquer cracks was noted in the age groups of 20 to 39 years. Klein and Curtin (27) reported that the mean age of the patients with lacquer cracks was 32 years with a range of 14 to 52 years. Tokoro (17) reported that the frequency of lacquer cracks was low in the patients younger than age 20 and in the elderly, but increases around ages 40 and 60 years. The frequency distribution of lacquer cracks showed two peaks in the age between 35–39 years and the age between 55–59 years.
Lacquer cracks might be a unique lesion among the various lesions of myopic maculopathy, because they seem to be caused almost purely by mechanical expansion of the globe and are not much influenced by aging. This is supported by the fact that chick models of experimental myopia which can develop axial myopia in 2 weeks of visual deprivation can develop lacquer cracks (28). Actually, lacquer cracks and subretinal bleeding caused by a new lacquer crack formation have been the only macular pathologies which reportedly develop in animal models of experimental myopia. This is also supported that lacquer cracks develop after LASIK surgery (29-33) or after laser photocoagulation (34).
In OCT examinations, lacquer cracks are observed as an increased light penetration into deep tissues with RPE discontinuity. The most recent technology; OCT angiography clearly visualized the crack of choriocapillaris.
It is uncommon for lacquer cracks develop across the central fovea. Thus, lacquer cracks themselves do not usually impair the central vision, however, the subretinal bleeding which develops at the onset of the rupture of BM could cause the impairment of central vision even after absorption of the hemorrhage.
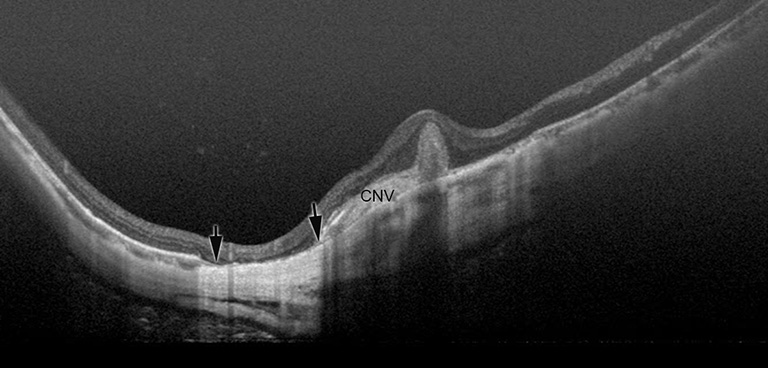
Myopic CNV is a major sight threatening complication of PM. It is the commonest cause of CNV in individuals aged below 50 years, and the second commonest cause of CNV overall (35,36). Myopic CNV tends to be small and thus about 20% of them was extrafoveal (37). Most of them are type II CNV, above the RPE. In natural course as well as after treatment, myopic CNV goes through three phases; active phase, scar phase, and atrophic phase (also known as myopic CNV-related macular atrophy) (Figure 7).
Since the introduction of anti-VEGF agents in ophthalmology around 10 years ago, anti-angiogenesis treatment with intravitreal anti-VEGF therapy has become the standard-of-care first-line treatment for myopic CNV (15,38). In addition, two large multi-centered, double-masked, randomized, controlled clinical trials have been performed to evaluate the use of anti-VEGF therapy for myopic CNV (39,40), the RADIANCE study (intravitreal injection of ranibizumab) and the MYRROR study (intravitreal injection of aflibercept). The results of these two large clinical trials were promising and significantly improved the visual outcome of the patients with myopic CNV.
However, it is not fully clarified whether anti-VEGF treatments are effective for late complications occurring around the scarred myopic CNV; which is CNV-related macular atrophy. Recent study using swept-source OCT by Ohno-Matsui et al. (41) showed that CNV-related macular atrophy was also an enlarged hole of BM around the CNV (Figure 5). To improve the long-term outcome of anti-VEGF therapies for myopic CNV, the prevention of BM hole enlargement around the CNV is necessary.
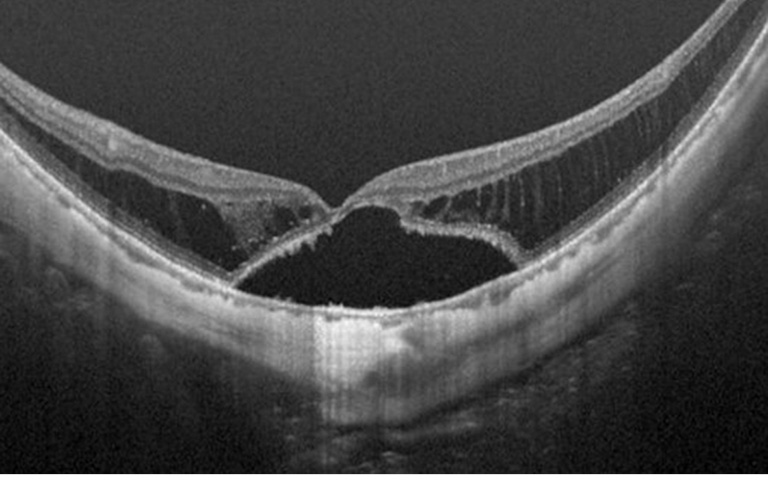
By using OCT, Takano and Kishi first demonstrated foveal retinal detachment and retinoschisis in severely myopic eyes with posterior staphyloma using OCT (42). Panozzo and Mercanti (43) proposed the term “myopic traction maculopathy (MTM)” to encompass various findings with traction in common by OCT in highly myopic eyes. OCT is an indispensable tool to diagnose MTM, which allows in vivo examination of macular morphology, such as schisis-like inner retinal fluid, schisis-like outer retina fluid, foveal detachment, lamellar or full-thickness macular hole and/or macular detachment.
Several mechanisms have been proposed for the development of MTM, including vitreomacular traction from partial posterior vitreous detachment (42,43), epiretinal membrane, intrinsic internal limiting membrane noncompliance (44), and retinal arteriolar stiffness (45).
Shimada et al. (46) have classified MTM according to its location and extent from S0 through S4: S0: no retinoschisis; S1: extrafoveal; S2: foveal; S3: both foveal and extrafoveal but not the entire macula; and S4: entire macula.
Due to the possible mechanisms involved in the pathogenesis of MTM, vitrectomy is the most common treatment to release all retinal tractions, which include cortical vitreous and epiretinal membrane. Special surgical technique; foveolar ILM (inner limiting membrane) sparing technique was used in an attempt to reduce the development of post-vitrectomy macula hole, which was a severe complication and resulted in poor visual recovery (47,48).
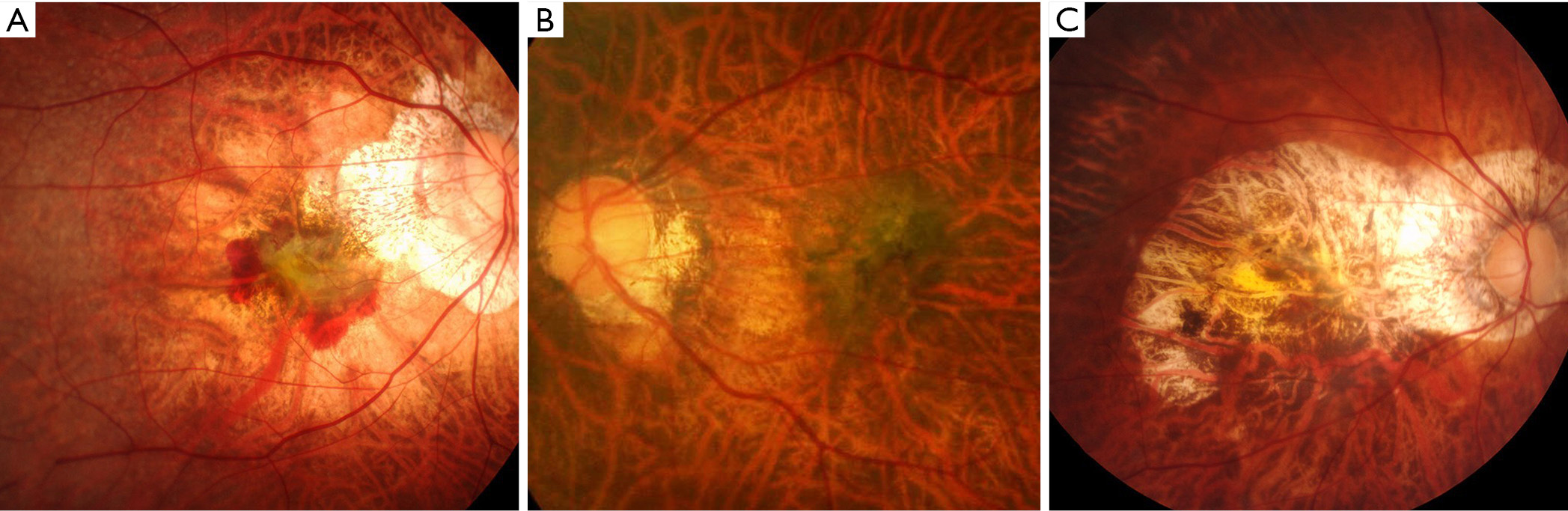
The results of earlier studies indicated that the high prevalence of glaucoma in highly myopic eyes was a great concern because the diagnosis of glaucoma was difficult in highly myopic eyes and thus it tended to be overlooked. The VF findings are difficult to interpret in eyes with PM. PM eyes usually have a large conus together with various extents of maculopathy, which make the analyses and interpretations of automated VF examinations difficult. Thus, most of the studies examining the prevalence of glaucoma have excluded highly myopic eyes. The papillary and peripapillary regions of highly myopic eyes are distorted by the mechanical stretching of the globe. The stretching results in the formation of various kinds of deformities of the optic discs (Figure 8) including tilted optic discs (49), acquired megalodiscs (50-52), and small discs. Nagaoka et al. (50) showed that HM eyes with megalodiscs had a 3.2 times higher risk of having glaucomatous optic neuropathy than HM eyes with normal sized or smaller optic discs.
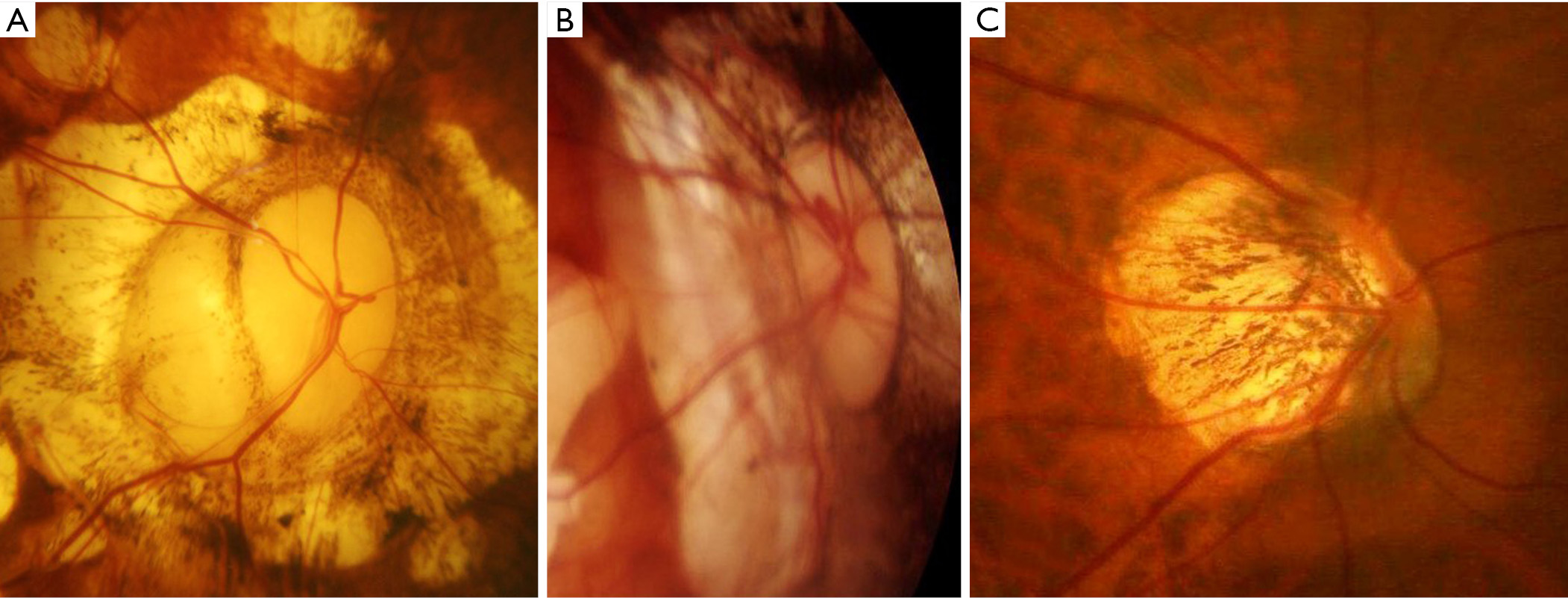
In addition to the mechanical deformity of the optic disc itself, other factors have been reported to be related to VF defects in eyes with PM. By using swept-source OCT, Ohno-Matsui et al. (53) reported that the optic subarachnoid space (SAS) appeared to be dilated in the eyes with PM. The dilated area exposed to the cerebrospinal fluid pressure along with thinning of the posterior eye wall may influence the formation of staphylomas and the way in which certain diseases, such as glaucoma, are manifested. In some cases, they also reported a direct communication between intraocular cavity and the SAS space (53).
Pit-like clefts at the outer border of the optic disc or within the adjacent scleral crescent have been observed in 16% of PM eyes but none in emmetropic eyes (54). The nerve fiber tissue overlying the pits was discontinuous at the site of the pits, and this might explain the cause of VF defects in highly myopic eyes in some cases. In addition, the loss of the nerve fiber tissue overlying peripapillary intrachoroidal cavitation (ICC) (55) was also observed in PM eyes, and it is considered as a cause of VF defects (56).
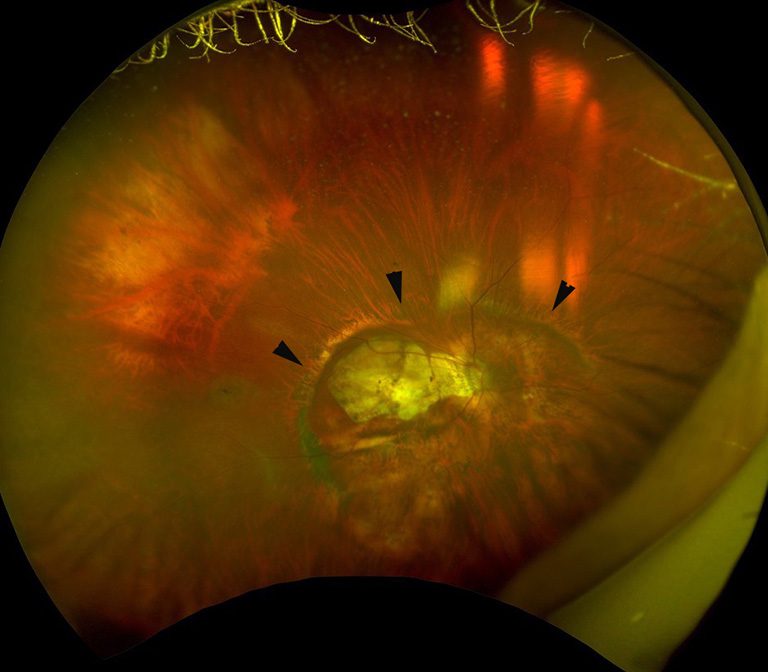
A posterior staphyloma is an outpouching of a limited area of the posterior segment of the eye (9), which is a representative deformity of eyes with PM. Posterior staphyloma is not a lesion of PM maculopathy but is a cause of complications occurring in the macula and in the optic nerve. Earlier studies showed that the presence of staphylomas was significantly correlated with worse vision, more frequent development of myopic macular complications, and optic nerve damage. The sclera protects the central nervous tissues, e.g., the neural retina and the optic nerve from mechanical insults. Thus, it is reasonable that a deformity of the eye by a staphyloma may result in a mechanical damage of the retina and optic nerve.
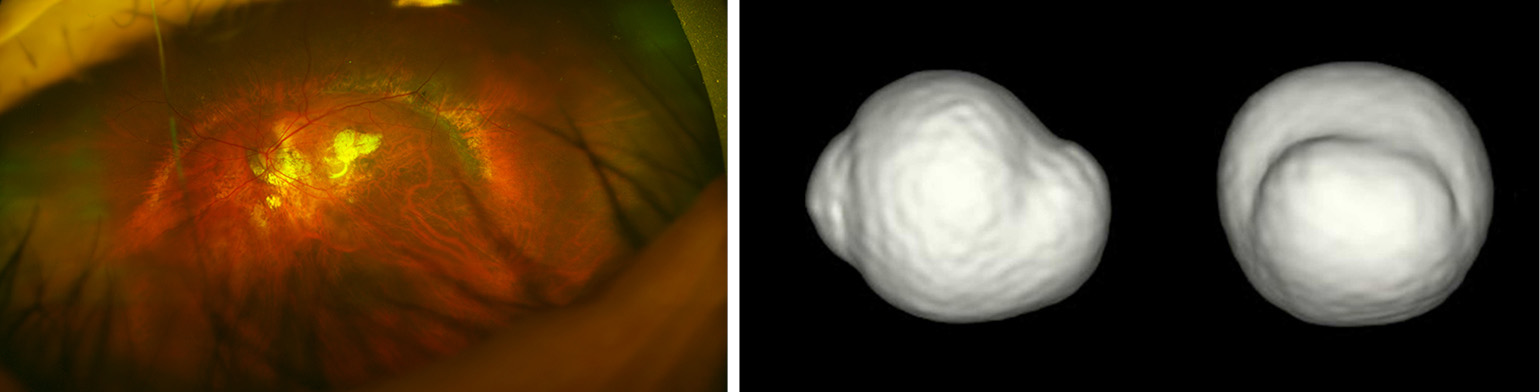
Based on ophthalmoscopic observation and fundus drawing, Curtin (7) first classified posterior staphylomas in eyes with PM into 10 different types based on ophthalmoscopy and fundus drawings. Types I to V were considered primary staphylomas, and types VI to X were combined staphylomas. Recently, new imaging modalities that can obtain images of the entire globe, e.g., 3D MRI (10,57,58) or ultra-wide fundus imaging (e.g., Optos) have become available. By using a combination of 3D MRI and Optos, Ohno-Matsui (58) (Figure 10) examined the prevalence and types of posterior staphylomas. These advances in ocular imaging have made the objective and quantitative evaluations of posterior staphylomas possible.
Thus, therapies targeting the sclera are considered potentially useful to prevent the development and progression of staphylomas. Posterior scleral reinforcement surgery for HM has been mainly performed in Russia and China, and some groups in the United States and Australia have also advocated scleral reinforcement for PM. Collagen cross-linking was recently introduced for the treatment of progressive keratoconus and post-refractive surgery corneal ectasia. Wollensak et al. (59) have pioneered the use of riboflavin and ultraviolet light (UVA) to cross-link collagen and enhance the mechanical properties of the cornea. The effect of scleral collagen crosslinking has been examined to treat myopia (60) and it is expected to be a future treatment to prevent staphyloma in PM. Recently, Shinohara et al. (61) reported that the transplantation of fibroblasts on the sclera would lead to the synthesis of collagen fibrils on the sclera and reinforce it, and reduce the degree of axial elongation of eyes with form deprivation myopia. Thus, regenerative therapies using patients’ own fibroblasts might be a promising approach to prevent posterior staphyloma, which is a cause of serious complications in PM.