Abstract: Optical coherence tomography (OCT) is an ocular imaging technique that can complement the neuro-ophthalmic assessment, and inform our understanding regarding functional consequences of neuroaxonal injury in the afferent visual pathway. Indeed, OCT has emerged as a surrogate end-point in the diagnosis and follow up of several demyelinating syndromes of the central nervous system (CNS), including optic neuritis (ON) associated with: multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), and anti-myelin oligodendrocyte glycoprotein (MOG) antibodies. Recent advancements in enhanced depth imaging (EDI) OCT have distinguished this technique as a new gold standard in the diagnosis of optic disc drusen (ODD). Moreover, OCT may enhance our ability to distinguish cases of papilledema from pseudopapilledema caused by ODD. In the setting of idiopathic intracranial hypertension (IIH), OCT has shown benefit in tracking responses to treatment, with respect to reduced retinal nerve fiber layer (RNFL) measures and morphological changes in the angling of Bruch’s membrane. Longitudinal follow up of OCT measured ganglion cell-inner plexiform layer thickness may be of particular value in managing IIH patients who have secondary optic atrophy. Causes of compressive optic neuropathies may be readily diagnosed with OCT, even in the absence of overt visual field defects. Furthermore, OCT values may offer some prognostic value in predicting post-operative outcomes in these patients. Finally, OCT can be indispensable in differentiating optic neuropathies from retinal diseases in patients presenting with vision loss, and an unrevealing fundus examination. In this review, our over-arching goal is to highlight the potential role of OCT, as an ancillary investigation, in the diagnosis and management of various optic nerve disorders.
Neuro-ophthalmology is a diverse discipline, which focuses on the relationship between neurological disorders and the visual system. The bedrock of diagnostic localization in neuro-ophthalmology consists of a thorough history and a complete clinical examination, without which ancillary tests have limited utility. Yet, advancements in ocular imaging technology, in the form of optical coherence tomography (OCT) have helped refine the diagnosis and management of various neuro-ophthalmic disorders, particularly the optic neuropathies. OCT can also be instrumental in distinguishing subtle retinal conditions from diseases of the optic nerve, especially in the context of a normal, near normal, or non-diagnostic fundus examination. In this review we will discuss the role OCT can play as a surrogate endpoint in diagnosing, following, and gauging response to therapy for a variety of optic neuropathies, including optic neuritis (ON), optic disc drusen (ODD), papilledema, and compressive optic neuropathy. We will also discuss potential future applications of OCT.
The structural effects of optic neuropathies have traditionally been identified through a detailed fundus examination, and include optic disc edema, optic disc pallor, and retinal nerve fiber layer (RNFL) defects (1). The RNFL, which contains the axons of the retinal ganglion cells, lacks myelin. Consequently, changes in RNFL structure, including slit or wedge defects, represent visible effects of axonal loss caused by retrograde degeneration, typically from an afferent visual pathway lesion involving the optic nerve, chiasm, or tracts (1). In the setting of trans-synaptic “neuroaxonal” (for the purposes of this review, “neuroaxonal” refers to retinal ganglion cells and their axons) degeneration, post-geniculate lesions in the afferent visual pathway can also cause optic nerve pallor, and corresponding RNFL defects, which can all be readily captured by OCT (1,2). Spectral domain OCT uses principles of low-coherence interferometry to acquire high-resolution (within 4–6 microns), images of retinal structural architecture in vivo (1). Emerging OCT techniques, such as swept source imaging, provide even better resolution, faster scanning, and repeatability (2). Recent advances in segmentation techniques (Figure 1) allow the thickness of individual layers of the retina to be quantified with OCT. This, in turn, can facilitate indirect quantification of axonal loss (RNFL thinning) and neuronal damage [ganglion cell-inner plexiform layer (GCIP) thinning] referable to various mechanisms of optic nerve, chiasm, and optic tract injury (1). Notably, OCT measured thinning of the macular GCIP has been found to have a strong relationship with visual loss across a spectrum of optic neuropathies including glaucoma, ON, ischemic optic neuropathy, hereditary optic neuropathy, toxic optic neuropathy, optic nerve glioma, and idiopathic intracranial hypertension (IIH) (2).
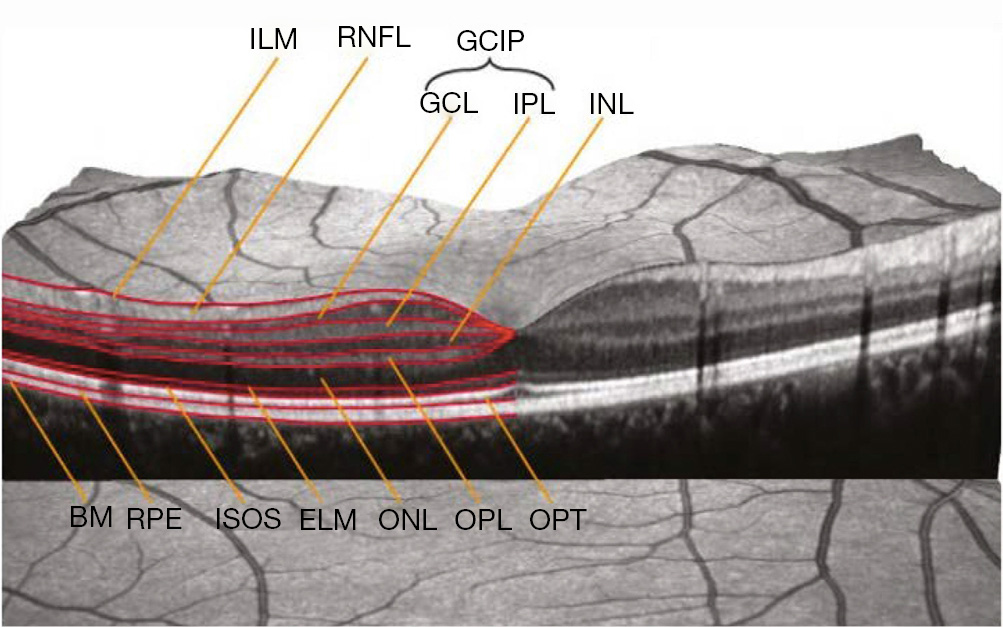
Yet, like any surrogate endpoint, the utility of OCT relies on our knowledge regarding the pathophysiology of the disease being followed; and, the mechanism of action of the intervention being evaluated (1). Moreover, the value of OCT to the neuro-ophthalmic evaluation is contingent upon our understanding of the inherent limitations and pitfalls associated with the use of this technology. Several reports have highlighted rates of false positive RNFL abnormalities (15–35%) in healthy adults (3), and the prevalence of OCT related imaging artifacts observed among patients (4). In particular, segmentation of the macula and measurements of the GCIP introduce significant error and misinterpretation, especially in eyes with pathology (e.g., age-related macular degeneration, optic disc edema) (2). Among healthy eyes, GCIP artifacts have been noted in 40% of cases (5). It is imperative to distinguish artifacts from true pathological changes with OCT, both to optimize the care of patients and obviate the need for invasive and costly investigations (2).
OCT first established a foothold in the field of neuro-ophthalmology, in the evaluation of ON and MS patients. In 1999, Parisi and colleagues used an early version of OCT to show that RNFL values are lower in MS patients with prior ON relative to healthy control subjects (6). Over the two decades since this seminal publication, there has been an influx of reports documenting the use of OCT in tracking the effects of ON and sub-clinical disease activity in MS patients. Moreover, OCT findings have been shown to be effective in distinguishing MS associated ON from optic nerve involvement in the NMOSD (7). The experience gleaned from studying patients with demyelinating syndromes of the CNS has shaped our understanding regarding how OCT measures can be used to capture the effects of CNS injury and repair (1).
ON is an inflammatory optic neuropathy commonly encountered in neuro-ophthalmic practice. This typically self-limited syndrome is often the presenting clinical complaint for MS patients, and is characterized by sub-acute onset vision loss and pain (8). Patients typically regain visual function within 3–6 weeks, whether or not they are treated with high dose corticosteroids (8). In the acute setting, ON is frequently characterized by a relative afferent defect in the affected eye, and a normal to slightly edematous optic nerve appearance (8). Visual field defects may be focal or diffuse. OCT measured RNFL values are initially elevated in acute ON, due to axoplasmic flow stasis in the inflamed optic nerve (1). This observation makes it difficult to rely on RNFL thickness to track structural changes in axonal integrity because it can often take 2–3 months for detectable RNFL thinning to manifest after ON (1). In this circumstance however, OCT measured GCIP changes represent an early, sensitive, and reliable sign of retrobulbar neuroaxonal injury (1). While OCT is not needed to diagnose ON, which can be determined in the absence of a surrogate endpoint, it may offer some prognostic information. More specifically, lower post-acute OCT measures correlate with reduced scores on high contrast letter acuity, low contrast letter acuity, color vision, and visual field sensitivity testing in ON eyes (9). To this end, OCT also can be used to interrogate structure-function relationship in the afferent visual pathway, and help us determine what factors contribute to recovery after MS related relapses.
Nearly all causes of ON lead to thinning of the RNFL and GCIP, yet the amount of loss can be helpful in differentiating the underlying etiology. Multiple studies have shown that ON eyes in NMOSD patients have more pronounced thinning of the peripapillary RNFL and macular GCIP than MS ON eyes (7). While anti-AQP4 antibodies account for the majority of NMOSD cases, anti-MOG antibodies have been shown to be present in approximately 25% of AQP4-seronegative patients with an NMOSD-like phenotype (10). When anti-MOG was compared to anti-AQP4 status in a series of patients with NMOSD, Martinez-Lapiscina demonstrated that patients with anti-AQP4 antibodies had significant thinning of the RNFL compared to MS-related ON patients, while anti-MOG patients were no different than their MS counterparts (11). In contrast, Pach et al. found no difference in the OCT measures when comparing patients with anti-MOG and anti-AQP4-mediated ON (12). While the latter study demonstrated substantial thinning of the RNFL and GCIP, the damage in the anti-MOG group was driven predominantly by more frequent ON attacks, whereas the anti-AQP4 group had more severe attacks leading to the structural OCT changes observed (12,13). It is also important to note that there is substantial overlap in the degree of thinning of the RNFL and GCIP between the various etiologies of ON and therefore OCT findings can provide supportive evidence of the cause of the ON, but cannot be interpreted in isolation for any given individual.
Lesions in the afferent visual system of MS patients are not limited to the optic nerve, but may also affect the optic chiasm, optic tracts, and retrogeniculate visual pathway regions. MS patients with retrochiasmatic lesions may report symptoms consistent with homonymous visual dysfunction, or may, alternatively, be unaware of the deficit. OCT can serve as a surrogate endpoint, or marker of relapse activity in this context because a pattern of hemi-retinal GCIP thinning (Figure 2) that corresponds to what is often a transient visual field defect, persists even after visual function has recovered. In a disease characterized by dissemination of CNS lesions in time and space, it is therefore foreseeable that OCT could complement existing criteria used to diagnose MS patients (14).
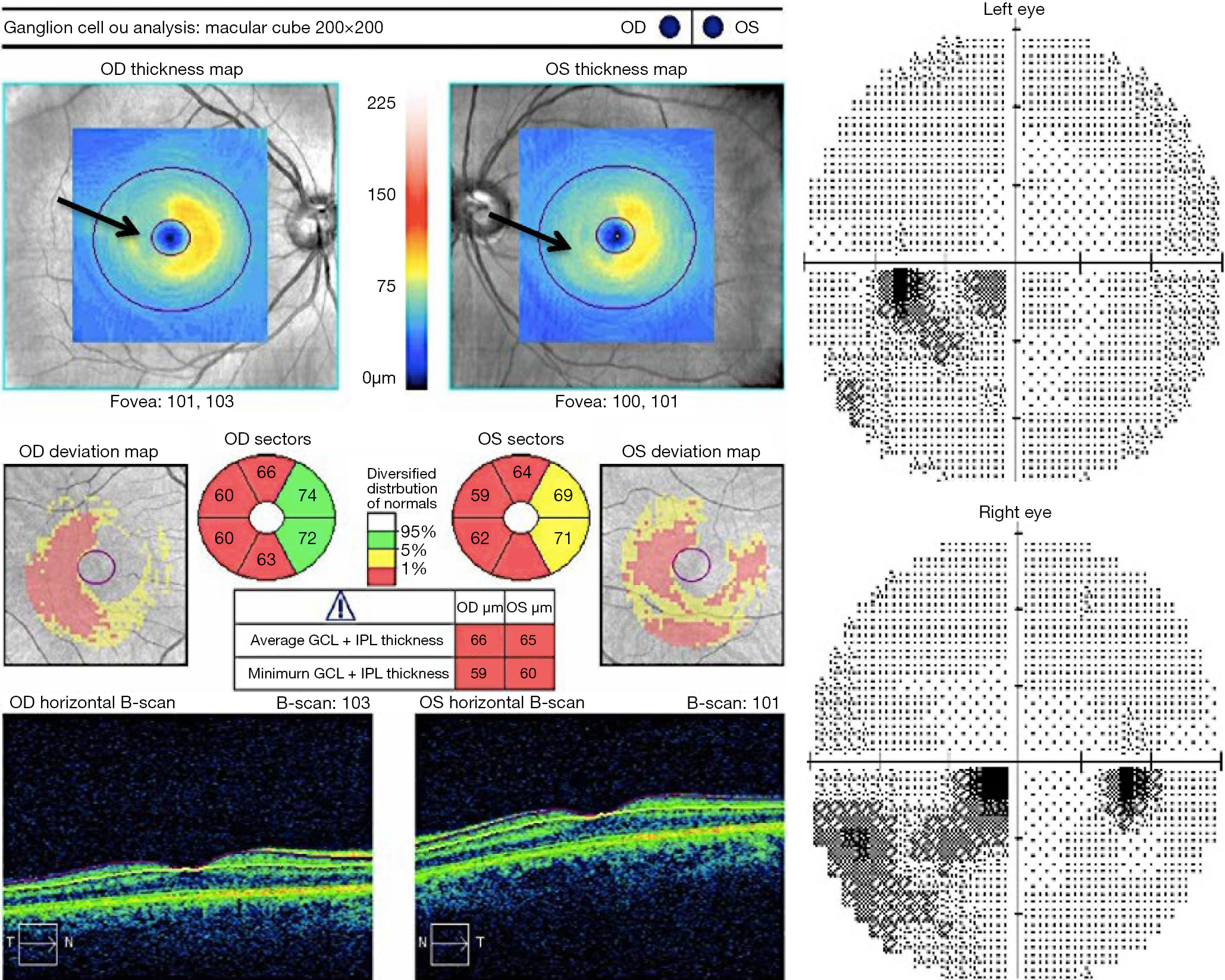
MS patients are known to experience substantial sub-clinical disease activity, which may be one reason why they harbor reduced OCT measures of RNFL thickness and GCIP thickness, even in the absence of prior ON events. In a recent meta-analysis, Petzold and colleagues compared 1,667 MS-ON eyes and 4,109 MS non-ON eyes to 1,697 eyes from healthy control subjects (15). Peripapillary RNFL values were thinner in MS-ON eyes [mean difference ?20 microns (μm), 95% CI, ?23 to ?17; P<0.0001] and in MS non-ON eyes (?7 μm, ?9 to ?6; P<0.0001), relative to healthy control eyes. OCT measured GCIP thinning was also prominent in both MS-ON eyes ?16 μm (?19 to ?14; P<0.0001) and MS non-ON eyes ?6 μm (?8 to ?5; P<0.0001)] relative to control eyes. The published work to date indicates that OCT can be used to quantify changes in neuroaxonal structural integrity that may reflect retrograde degeneration from lesions anywhere along the course of the afferent visual pathway of MS patients. Furthermore, reduced RNFL and GCIP values have been shown to correlate with other surrogate endpoints used to monitor disease activity including: MRI-measured brain atrophy, MRI measured T2- and gadolinium-enhancing lesions, neurological disability scores, and clinical relapses (1,9).
The current goal of treatment in MS is referred to as “no evidence of disease activity” or “NEDA”. The most commonly used form of NEDA is referred to as NEDA-3, and is characterized by the absence of: relapses, Expanded Disability Status Scale (EDSS) progression, and T2/gadolinium-enhancing lesions as detected by MRI (16). In a recent report, Pisa and colleagues followed RNFL values in MS patients over 2 years, and attempted to stratify individuals based upon whether or not they met NEDA-3 criteria (17). These investigators observed that the rate of OCT-measured RNFL thinning in NEDA-3 patients was ?0.93 μm (±1.35 SD) while in non-NEDA patients it was worse, measuring ?2.83 μm (±2 SD). It is noteworthy that in this study NEDA patients had less RNFL thinning than non-NEDA patients, yet both groups had pathological rates of RNFL loss when compared to normal individuals (?0.16 μm/year) (17).
Recent interest in the field of MS has focused on the significance of OCT-detected microcystic macular edema, and inner nuclear layer (INL) thickness in these patients. Microcystic macular edema has been reported to affect approximately 5% of MS patients, and is characterized by discrete microcysts typically found within the INL (18,19). It has been postulated that microcystic macular edema in MS eyes represents a breakdown of the blood-retinal barrier, and integrity of tight junctions in the myelin-free retina. It has also been observed that microcysts are more frequently observed in NMOSD associated ON, as compared to MS associated ON (7). Yet, microcystic macular edema is by no means unique to MS, NMOSD or inflammatory optic neuropathies as a whole, but can be observed across a wide-variety of ophthalmic conditions (20). Caution must be therefore exercised when making inferences regarding the significance of microcystic macular edema, and by extension, transient fluctuations in INL thickness in MS patients, particularly in the absence of a detailed clinical ophthalmic evaluation.
Key points: OCT as a surrogate endpoint?
ODD are acellular, hyaline deposits located in the optic nerve, which often become calcified over time (21,22). Previous reports have estimated that ODD affect approximately 3% of the general population (21,22). While initially felt to be bilateral in 75% of cases (21,22), recent work by Malmqvist and colleagues has shown that ODD are a bilateral phenomenon in approximately 95% of cases (23).
ODDs are believed to arise as a consequence of impaired axonal metabolism in genetically predisposed patients, who may (or may not) have narrow scleral canals (21,22). This axonal disruption hypothesis is germane to OCT interpretation, because ODD need to be differentiated from other optic nerve diseases, which can also impair axonal function. While many patients with ODD report no symptoms whatsoever, visual field defects are noted in the majority of affected eyes, particularly in the setting of superficial optic nerve head drusen (21). Patients with ODD can also develop acute secondary manifestations of vision loss due to nonarteritic AION, choroidal neovascular membrane, and retinal vascular occlusions (24).
Ancillary testing is generally not needed to detect superficial ODD, located on the surface of the optic nerve. In this setting the fundus examination will often reveal an elevated optic nerve head, with blurred disc margins, and a nodular appearance of the disc border (25). When ODDs are buried within the body of the nerve, however, the optic disc appearance can be confused with other causes of optic disc elevation, including papilledema. For this reason, additional investigations are necessary to diagnose ODD, and to exclude potentially life-threatening causes of raised intracranial pressure. Investigations that have been used in the diagnosis of ODD include fundus autofluorescence, computed tomography, fluorescein angiography, and B-scan ultrasonography (21). Specifically, B-scan ultrasonography has been viewed as the “gold standard” test for the detection of buried ODD (21). With this noninvasive technique, calcified ODD appears as hyperechoic, highly reflective round structures that can be distinguished by their posterior acoustic shadowing (21). Yet, detection of ODD with B-scan ultrasonography is dependent on the property of calcification, therefore, non-calcified ODD may be missed (21). Moreover, ultrasonography offers relatively poor resolution of drusen structures, and imparts little understanding regarding the structural integrity of the RNFL or retinal ganglion cell population in ODD eyes. Hence, ultrasound can facilitate the diagnosis of ODD, but cannot be used to follow drusen progression, or to predict visual outcomes in patients affected by this condition.
The OCT characteristics of ODD have been variably represented in the published literature, largely due to reliability issues inherent to older versions of OCT. In a prior spectral-domain OCT study, the diagnostic accuracy of ODD detection by five experienced clinicians was relatively modest, ranging from 50% to 64% (26). Importantly, in this study, several eyes with papilledema were mistaken for buried ODD or normal appearing optic nerves (26). Mild thickening of the peripapillary RNFL as measured by OCT, is also not a helpful means of differentiating cases of early papilledema from pseudopapilledema caused by ODD, because this finding can be observed in both clinical scenarios. Kulkarni et al. found no difference in RNFL thickness in any of the four quadrants between eyes with buried ODD and mild papilledema (26). These observations were significant because the investigators specifically focused on comparing buried ODD eyes to those affected by mild papilledema (with less than or equal to Frisén grade 2), which is the diagnostic conundrum most likely to challenge physicians who would otherwise have no difficulty distinguishing obvious ODD from higher grades of papilledema in patients with raised intracranial pressure.
In prior studies using earlier versions of OCT technology, ODD have sometimes been described as having a signal-poor core surrounded by a hyper-reflective margin. In these reports, the integrity of the hyperreflective margin has often been incomplete, with poorly demarcated anterior borders, and absent posterior signal (21,22). These technical issues have been caused by poor penetrance, a limitation, which has hampered the interpretation of earlier spectral domain and time domain OCT studies in ODD patients (21). In 2011, Lee and colleagues reported that ODD appeared as a highly reflective round sub-retinal structure with a well-defined margin that displaced the adjacent tissues (27). Previous studies have also inadvertently characterized peripapillary hyper-reflective ovoid structures (PHOMS), as ODD (21). Subsequent work has shown that PHOMS represent disruption of retinal layers caused by local axoplasmic build-up (21). It has been demonstrated that PHOMS are not unique to ODD eyes, but are also found in patients with optic disc elevation caused by other optic nerve disorders, including optic nerve tumors (28). Earlier OCT studies have also suggested that a “lazy V” pattern or “lumpy bumpy” internal contour of the subretinal hyporeflective space (29,30) represent features to help identify ODD, and rule out of papilledema. Yet, the so-called subretinal hyporeflective space represents a by-product of the poor penetration inherent to prior generations of OCT (21). Issues with poor penetration also may have contributed to the exceedingly high number of drusen cases reported in a recent study by Birnbaum and colleagues (31). In this retrospective review, the prevalence of ODD (19%) among eyes with resolved papilledema was approximately 10 times higher than that of the general population.
The debut of commercially available EDI OCT technology has enabled penetration and visualization of structures 500–800 μm deeper than previously possible with conventional OCT (21). For this reason, it is now possible to quantify drusen size, delineate their borders, and to assess the integrity of adjacent retinal structures. With EDI-OCT, ODD typically appear as signal-poor core surrounded by a hyperreflective margin (Figure 3) (21). Merchant et al. compared EDI-OCT to conventional diagnostic methods, including B-scan ultrasonography, used to detect ODD in eyes with definite ODD, suspected ODD, and normal appearing optic nerves (32). EDI-OCT had a significantly higher drusen detection rate than B-scan ultrasound (32). More recently, Traber et al. identified three morphologic ODD types with EDI-spectral domain OCT including: peripapillary subretinal drusen, granular drusen, and confluent drusen (33). In this study, the size and type of ODD as classified by OCT morphologic characteristics were significantly different in patients with or without visual field defects. Specifically, these investigators observed that confluent, large, and autofluorescent ODD were more commonly found in patients with visual field loss (33). Other EDI-OCT reports have suggested that ODD volumes correlate with structural optic nerve head damage, and functional deficits among patients (34). With current OCT technology, it is possible to devise three-dimensional images of ODD, such that quantification of its drusen volume can be monitored over time. Going forward, we will have the ability to detect longitudinal changes in ODD structure, and associated neuroaxonal changes in the retina. As a result, we may be able to use OCT to identify risk factors and potentially provide prognostic guidance regarding vision loss in ODD patients.
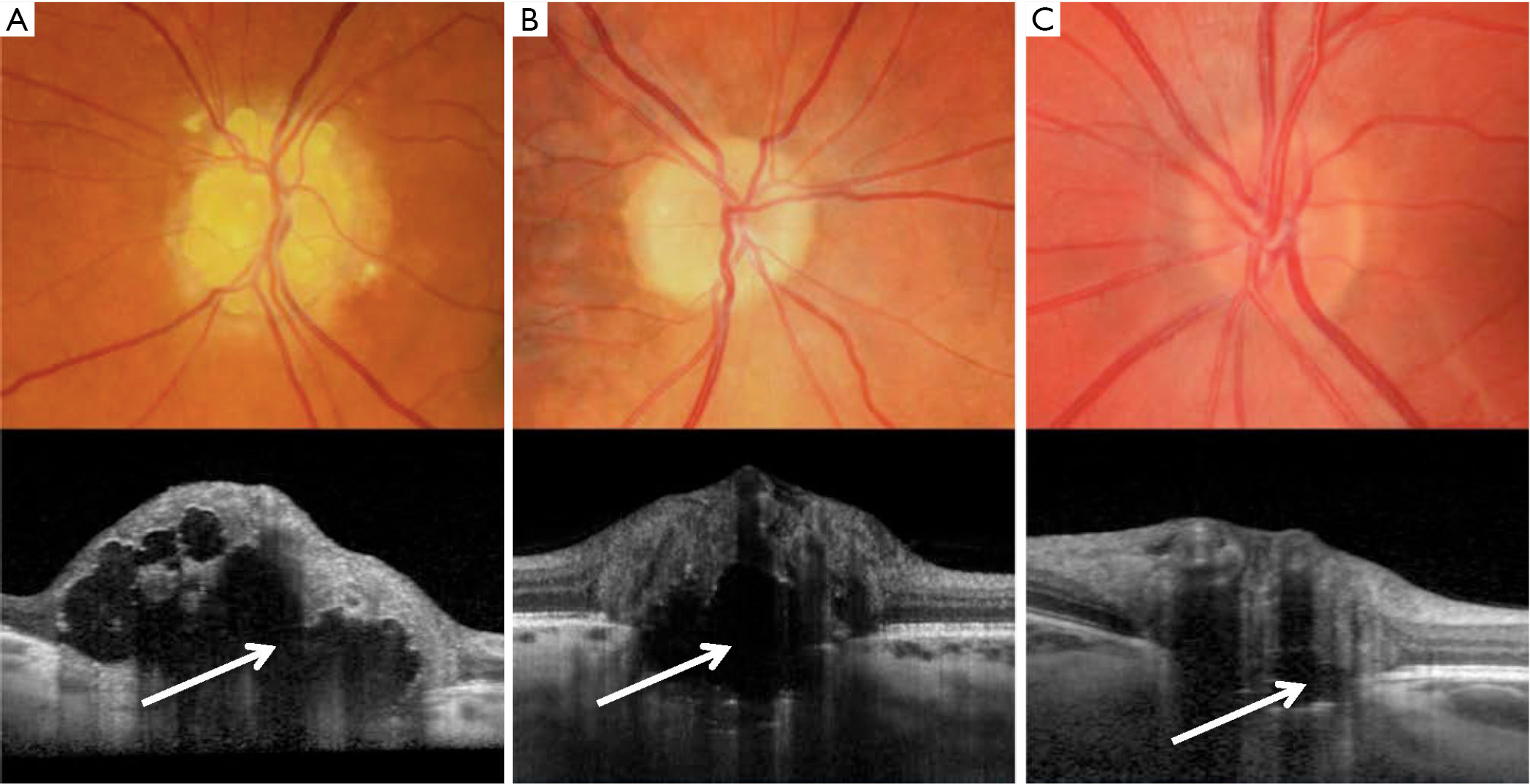
Key points: OCT as a new diagnostic gold standard?
IIH is a condition of increased intracranial pressure of unknown cause producing headaches, papilledema, and visual loss (35-37). IIH typically occurs in obese women in the childbearing years. Because of the recent obesity epidemic, the incidence of IIH has doubled over the past 20 years (38). While this condition was once called benign intracranial hypertension, the term “benign” is no longer used because these patients have the risk of severe visual impairment (39,40). Permanent visual loss can be seen in up to 40% of patients (40,41), and therefore affected individuals need close monitoring of the vision and visual fields.
OCT can play an important role in monitoring the disease process in IIH (42). Following the peripapillary RNFL thickness over different visits provides a quantitative and sensitive measurement of changes in the papilledema. While the Frisén grading scale for clinical papilledema ranges from grade I to V, the peripapillary RNFL thickness can range from 50 μm to over 500 μm giving it a much larger dynamic range to objectively evaluate for change. In addition, there is significant variability even among experts in grading papilledema based on the Frisén scale, and therefore the peripapillary RNFL is easier to follow objectively for change, especially when the patient is seen by different care providers (43-45).
A reduction in peripapillary RNFL thickness in the eyes of IIH patients can be either a result of improving papilledema or worsening axonal loss from disease progression. Combining the macular GCIP thickness with the peripapillary RNFL thickness allows one to evaluate for optic neuropathy in the presence of papilledema (46-49). The ability to monitor the integrity of the optic nerve in the setting of papilledema is arguably the most compelling reason to use OCT for monitoring IIH. Successful treatment with protection of neuroaxonal structure will cause a reduction in the peripapillary RNFL thickness with a preserved macular GCIP thickness. However, a concordant reduction in the RNFL thickness and macular GCIP thickness indicates worsening optic neuropathy and could be an indication of treatment failure or fulminant IIH (47). Therefore, combining the peripapillary RNFL analysis with the macular GCIP analysis allows one to differentiate treatment response from worsening disease. This is especially important in the setting of patients with functional overlay, which is not uncommon in IIH (50), or in patients unable to perform visual fields due to age or mental capacity; OCT provides an objective measurement of the structural integrity of the optic nerve, allowing ready detection of worsening optic neuropathy (42). However, it is important to note that there can be errors in segmentation of the GCIP in the setting of high degrees of papilledema. Segmentation errors will lead to a falsely thin GCIP thickness and could raise false alarm of worsening disease if they are not recognized. Fortunately, these artifacts can be easily identified because they result in significant reductions in the GCIP thickness in a non-physiologic pattern (2).
The configuration of Bruch’s membrane, as detected with OCT, has been shown to correlate with intracranial pressure and therefore is another feature that can be followed in IIH patients (Figure 4) (51,52). Patients with raised intracranial pressure have an upward defection of Bruch’s membrane toward the vitreous, which can be helpful in differentiating papilledema from pseudopapilledema. Kupersmith, Sibony, and colleagues have found that treatment of papilledema will lead to normalization of Bruch’s membrane complex orientation toward the posterior direction (51,53). This observation was confirmed in the IIH Treatment Trial, in which patients treated with acetazolamide had a significant posterior displacement of Bruch’s membrane (54). While changes in the fundus appearance of papilledema typically take days to develop, alterations in the Bruch’s membrane complex configuration occur more rapidly and can be seen within an hour of cerebrospinal fluid lowering procedures. It has been shown that OCT testing performed immediately after lumbar puncture-induced lowering of the intracranial pressure shows measurable posterior defection of Bruch’s membrane, even in the absence of detectable changes in peripapillary RNFL thickness (55,56). Therefore, clinically relevant changes in intracranial pressure, caused for example by shunt failure, could possibly be best detected by evaluation of the Bruch’s membrane complex morphology. This OCT feature could be particularly useful in assessing patients with pre-existing optic atrophy, among whom RNFL values (and fundus examination of the optic nerve) may not be a reliable means of tracking worsening elevations in intracranial pressure.
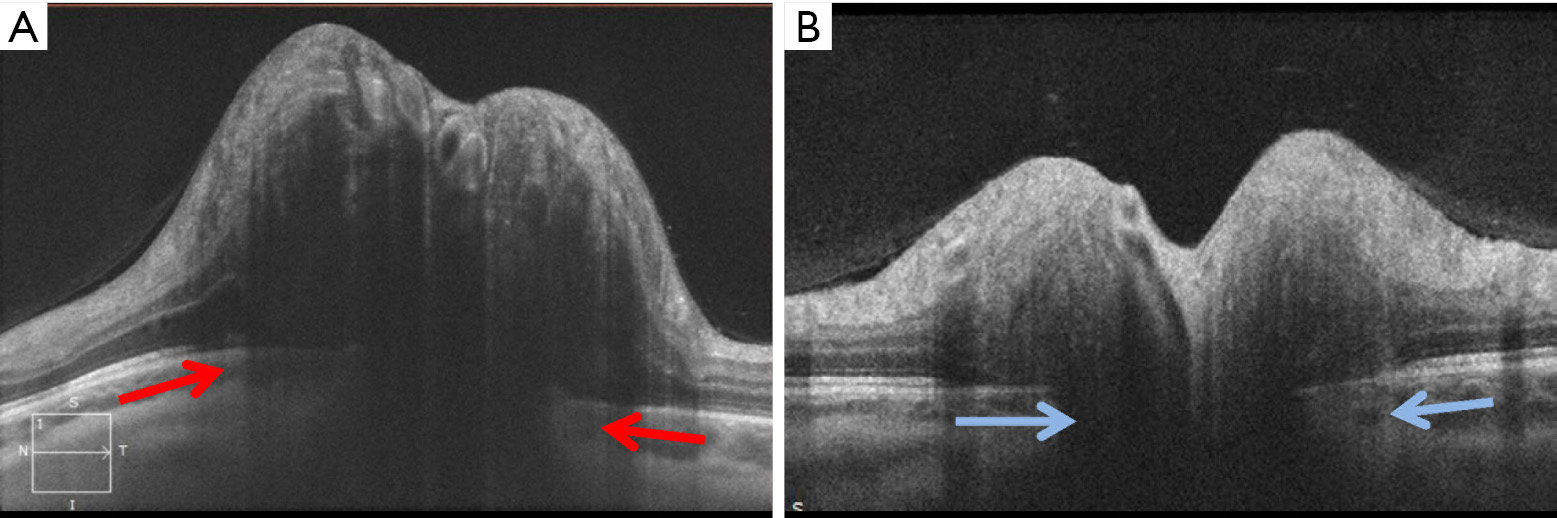
Lastly, OCT can detect retinal causes of vision loss from IIH, such as subretinal fluid or choroidal folds (42,45,57). This application has an important clinical role, because patients with decreased vision from optic neuropathy secondary to severe papilledema require aggressive treatment, while decreased vision due to subretinal fluid or choroidal folds is typically more benign (47,57-59). Studies by Chen et al. and Hoye et al. demonstrated that the majority of patients with visual acuity loss from subretinal fluid alone can be medically managed, with good visual outcomes despite high grade papilledema (47,57).
Key points: OCT to manage papilledema
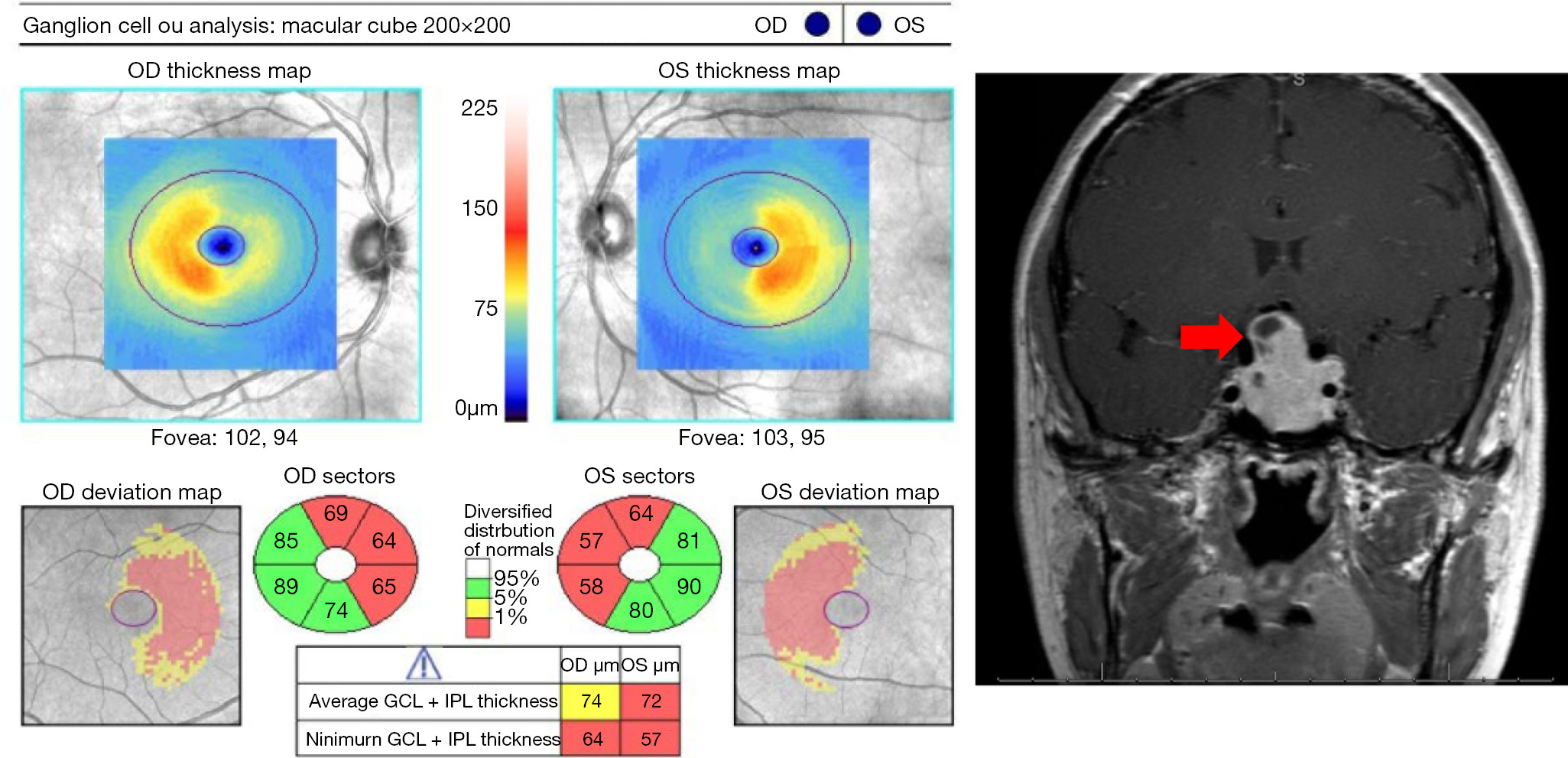
Slowly progressive vision loss suggests a cause of compressive optic neuropathy, until proven otherwise. This is an important clinical pearl to consider, because surgical decompression can lead to significant visual recovery if compressive lesions are detected early. The most common causes of compressive optic neuropathy include meningiomas, thyroid eye disease, and pituitary lesions. Compression of the optic nerve or chiasm leads to optic atrophy over time, which can be easily visualized on OCT. In this setting, OCT serves two roles: diagnosis of compression; and, prediction of visual outcomes after decompression. Subtle optic nerve damage from compressive optic neuropathy can be appreciated on OCT before it can be seen on ophthalmoscopy. This is especially true for the macular GCIP analysis, which is very sensitive for detecting compressive optic neuropathy. With compression of the optic nerve, macular GCIP thinning precedes peripapillary RNFL loss (60), and occasionally, GCIP thinning can be seen even before appreciable changes are detectable with standard automated perimetry (61-63). However, visual field loss can also occur before OCT changes in early compressive optic neuropathy, and therefore visual fields remain a necessary part of the workup in patients with potential compression of the optic nerve apparatus (64).
While some anterior compressive lesions, such as optic nerve sheath meningiomas, will cause visible disc edema, other compressive lesions will sometimes cause mild axoplasmic flow stasis leading to subclinical RNFL thickening that can be seen on OCT, but may not be visible on ophthalmoscopy. In early compression, this can lead to a thickened RNFL compared to age-matched controls. After further optic nerve damage and consequent atrophy, this will often lead to a “normal” RNFL thickness despite significant macular GCIP thinning. Further compression then leads to both RNFL and GCIP thinning. This subclinical RNFL thickening is not unique to compressive optic neuropathy and is also seen in other optic neuropathies, such as Leber hereditary optic neuropathy (LHON) or retrobulbar ON, but this OCT appearance in the setting of slowly progressive vision loss is suggestive of compressive optic neuropathy and warrants neuroimaging.
The pattern of macular GCIP loss is helpful in detecting some compressive optic neuropathies, especially if they involve the chiasm or optic tract. The macular GCIP analysis is exquisitely sensitive in detecting chiasmal injury, which causes binasal thinning because the crossing nasal fibers are damaged with compression of the chiasm (Figure 5) (62,63). This OCT feature can be valuable in differentiating chiasmal compression from other optic neuropathies, such as glaucoma, which tends to cause thinning of the macular GCIP that respects the horizontal meridian. Diffuse thinning of the macular GCIP in one eye with nasal thinning in the contralateral eye will be seen in a junctional scotoma from anterior compression of the optic chiasm (62). While binasal ganglion cell loss is seen in chiasmal injury, homonymous macular ganglion cell loss typically indicates injury to the optic tract or lateral geniculate nucleus from various etiologies, including tumor, stroke, or demyelination (Figure 2) (65). As discussed previously, post-geniculate lesions can also cause homonymous ganglion cell layer thinning through trans-synaptic retrograde degeneration (66-68), but this OCT pattern likely takes at least a year to develop.
In addition to diagnosing compressive lesions, OCT plays a role in predicting visual outcome after surgical decompression. Both peripapillary RNFL and macular GCIP thicknesses have been shown to correlate with visual field loss from compressive lesions and also correlate with visual outcomes after surgical decompression (62,69-73). If there is prominent preoperative peripapillary RNFL and macular GCIP thinning, there is a lower chance of complete recovery, even after surgical resection and resolution of the compression on the nerve or chiasm. However, previous reports have highlighted cases of “complete” recovery of visual fields despite pre-operative thinning of the RNFL and GCIP detected with OCT. Most likely, this represents a lack of sensitivity of standard automated perimetry in identifying subtle residual visual field loss. This is supported by a patient described by Horton, who had a normal visual field with conventional size III stimulus after surgical resection of a pituitary adenoma, but was noted to have a mild persistent bitemporal hemianopia on visual fields with the smaller size I stimulus (74).
Several studies have suggested that the preoperative macular GCIP thickness may better predict visual outcomes after surgical decompression than the peripapillary RNFL (62,72,73). However, others have suggested that RNFL may better predict surgical outcomes after surgery (60,75). There is no doubt that the macular GCIP is more sensitive in detecting compression, but the prediction of visual outcomes is likely dependent on multiple factors, including the location, duration, and severity of compression. For example, anterior lesions can cause axoplasmic flow stasis and RNFL thickening that masks optic atrophy, and therefore outcomes may be better predicted by the macular GCIP in these cases. Alternatively, surgical outcomes for early compression may be better predicted by RNFL thickness because GCIP thinning may be too sensitive and be detected in patients with normal visual function. The combination of peripapillary RNFL and macular GCIP is most helpful and ideally should both be obtained in cases of suspected compressive optic neuropathy. Although there is variability, OCT-derived measurements of the optic nerve can provide some guidance as to the expected outcome of surgery for both the physician and patient.
Key points: OCT can be used to redefine structure-functional relationships
All optic neuropathies lead to optic nerve pallor, which can be objectively measured as peripapillary RNFL and macular GCIP thinning. In fact, OCT will detect damage from remote optic nerve injury, including for example, prior ON with complete visual recovery (76,77). Furthermore, OCT is helpful in detecting subtle optic neuropathies, such as LHON (78,79). Patients with LHON can sometimes be incorrectly diagnosed with functional vison loss because the optic nerve changes can be subtle, especially early in the disease. Progressive macular GCIP thinning manifests over 6 months and can be detected even before the onset of vision loss (78). The peripapillary RNFL will remain normal to slightly thickened for 3–6 months because of the pseudo-edema that is seen in this condition.
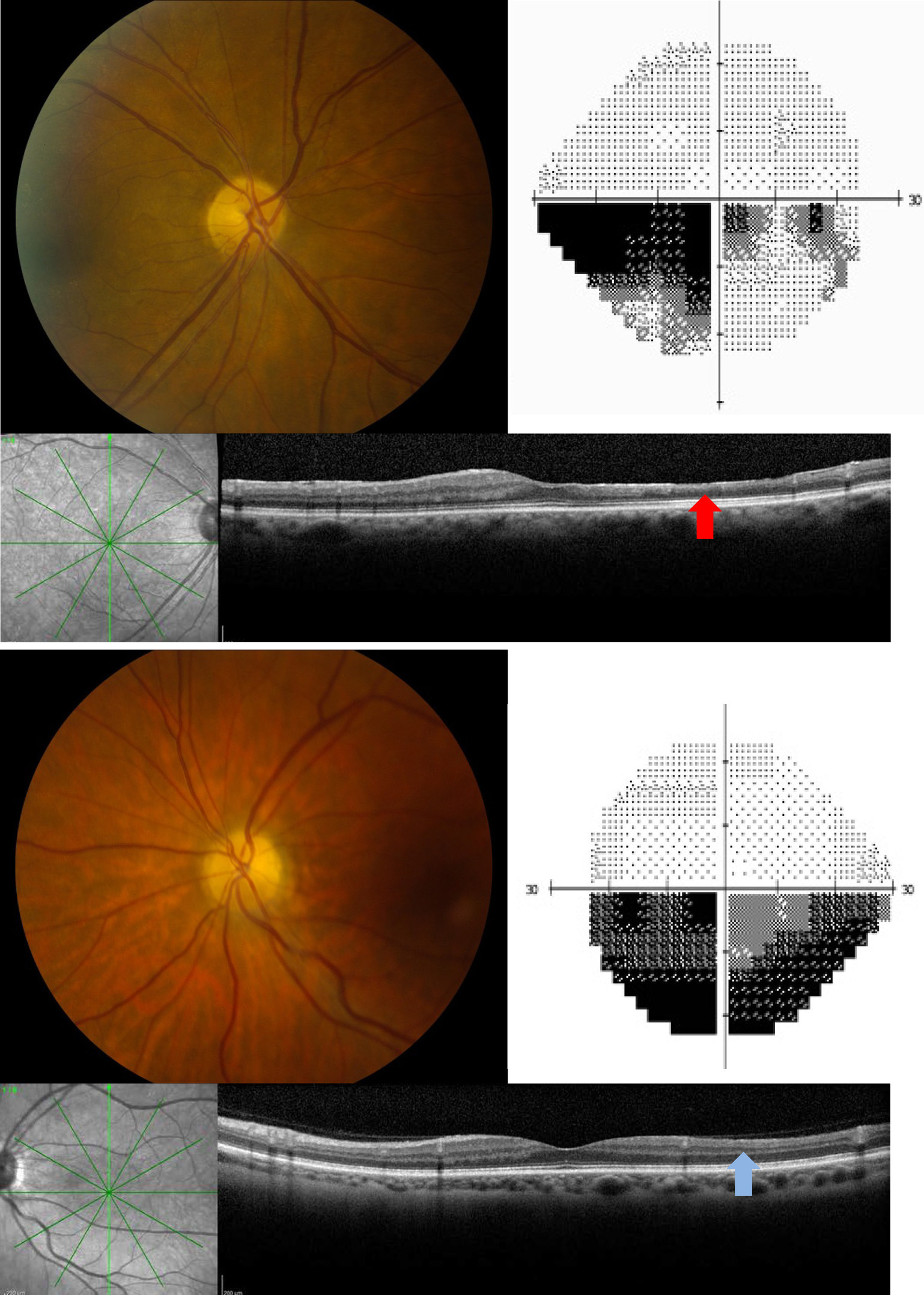
OCT can be invaluable in identifying subtle retinal pathologies that can masquerade as optic neuropathy. Retinopathies affecting the photoreceptors, such as cancer associated retinopathy, autoimmune retinopathy, retinitis pigmentosa sine pigmento, and acute zonal occult outer retinopathy, can all cause significant visual field loss without abnormalities on ophthalmoscopy, especially early in the disease course. Typically, OCT will show loss or disruption of the ellipsoid zone corresponding to the outer segments of the photoreceptors that matches the visual field loss (Figure 6). This is an incredibly easy and efficient way of diagnosing photoreceptor disease and directing the subsequent workup. However, it is important to note that rarely the ellipsoid zone will be intact on OCT analysis, despite vision loss from a subtle retinopathy. In these cases, electroretinography may still be required to make the diagnosis. Since photoreceptor loss, macular edema and subretinal fluid are easily detected with OCT, the technology can play an important role in the diagnosis of conditions such as central serous retinopathy, in which visible fundus changes from subretinal fluid can be subtle. Fingolimod, a commonly used medication for MS patients, can rarely cause cystoid macular edema, which is readily appreciable on OCT (80,81).
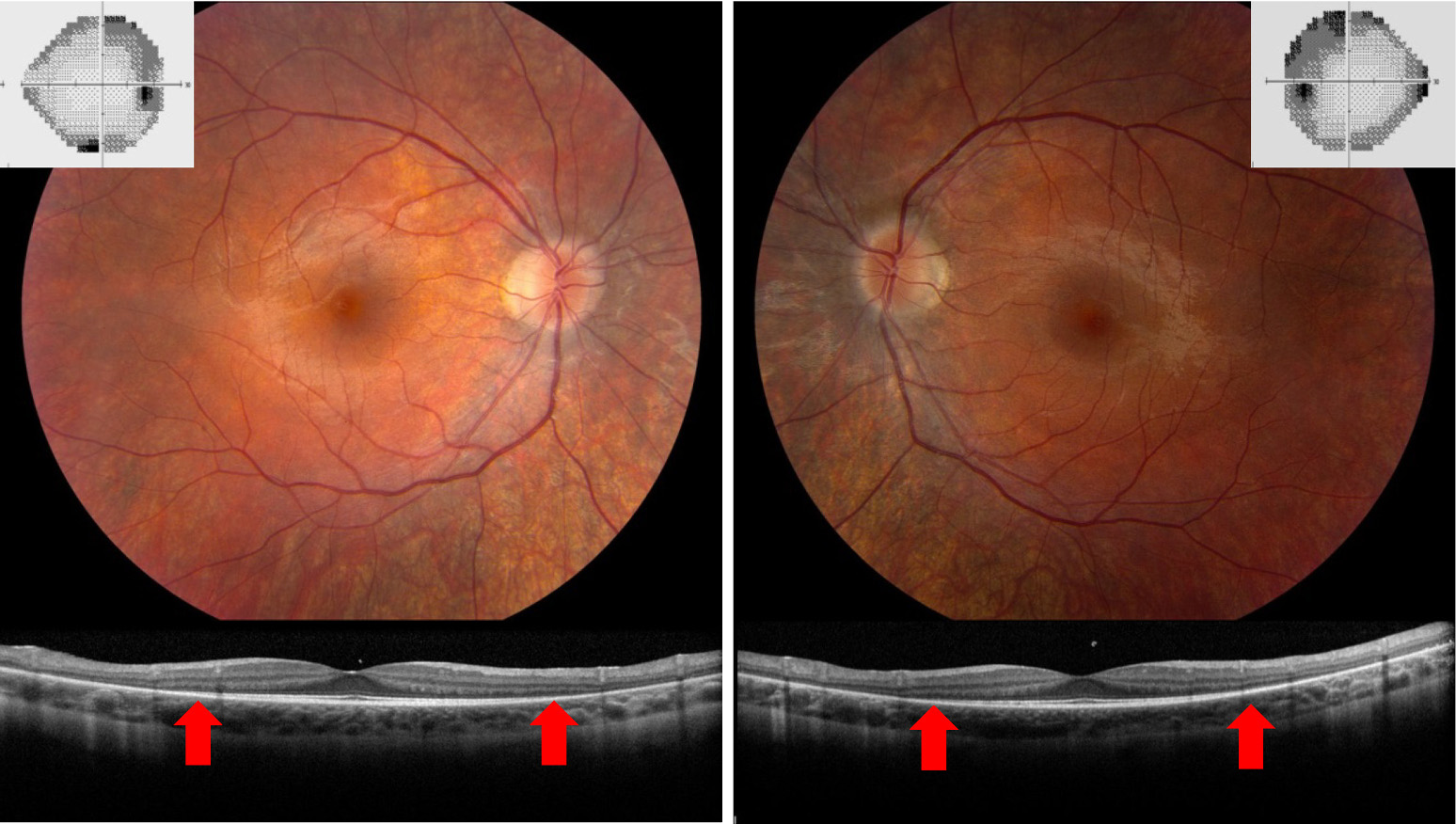
OCT can also be used to distinguish retinal artery occlusions from forms of optic neuropathy, such as nonarteritic AION. Because the central and branch retinal arteries supply the inner retina, retinal artery occlusions will affect the inner retina and cause structural changes that can be seen on OCT. In the acute setting, retinal whitening and thickening of the inner retina is visible on OCT after retinal artery occlusions. In the chronic setting, retinal artery occlusions will cause thinning of the entire inner retina, including the INL. In contrast, optic neuropathies will cause thinning that is limited to the RNFL and GCIP layers. These OCT patterns can be used to differentiate a chronic branch retinal artery occlusion from nonarteritic AION, both of which can cause an altitudinal defect and corresponding pallor of the optic nerve in the post-acute phase (Figure 7). This is an important clinical distinction because retinal artery occlusions require a thromboembolic workup, whereas cases of nonarteritic AION do not.
Lastly, OCT is an objective measurement that is helpful for providing reassurance in the setting of functional vision loss. As discussed above, OCT is exquisitely sensitive in detecting subtle optic nerve or retinopathy pathology. Therefore, OCT is a helpful adjunct test for ruling out organic disease and can be useful during the discussion with a patient with functional vision loss. However, it is important to note that OCT is a structure measurement and can be normal in the setting of acute vision loss from an optic neuropathy, and therefore repeat OCT testing over time is sometimes warranted to rule out true organic disease.
Key points: OCT—is it optic nerve, retinal, or functional?
OCT technology is continuing to improve, providing higher resolution and new functionality. Early time domain OCT has been supplanted by spectral domain, a generation of OCT that brought markedly improved scan speeds and resolution. Recently, OCTA and swept source OCT have been introduced, and these advancements will undoubtedly have an expanding future role in neuro-ophthalmology.
OCTA provides the capability of imaging the capillary network in the retina, which is not visible with traditional fluorescein angiography (82) OCTA is achieved by obtaining rapid sequential scans and evaluating for changes in the images to detect blood flow, which is then used to construct microvascular density maps (82-85). Multiple studies have shown decreased optic disc perfusion and peripapillary capillary density on OCTA in patients with chronic glaucoma (84,86-89). There has been debate as to whether the changes seen on OCTA are ischemic changes from glaucoma, or secondary to the glaucomatous damage (90).
The role of OCTA in neuro-ophthalmology is slowly growing. In MS patients, OCTA has shown decreased vessel density and reduced blood of the optic nerve head (optic nerve head flow index) in eyes with and without a history of ON (85,91). OCTA has also been shown to detect early vascular changes in LHON (92-95). In these cases, OCTA demonstrates peripapillary telangiectasias and capillary dilation in eyes with conversion and acute vision loss, whereas in sub-clinical eyes, OCTA can show subtle dilation in peripapillary vessels (92). Over time, there is peripapillary capillary loss that mirrors the RNFL loss seen in LHON eyes (93). Because nonarteritic AION is an ischemic process, there has been hope that OCTA would provide insight into underlying pathogenic mechanisms in this condition. Some studies suggest that a decrease in peripapillary capillary perfusion can be seen in acute nonarteritic AION (96-98), but it is important to note that the disc edema in this context can cause some artifact on OCTA. In chronic nonarteritic AION, studies have shown decreased peripapillary capillary density on OCTA that correlates with RNFL thinning and visual field loss (99-101).
Interestingly, it was recently shown that all chronic optic neuropathies, including etiologies that are not thought to be vascular in nature (including ON and traumatic optic neuropathy), show decreased peripapillary capillary density on OCTA that correspond to areas of RNFL thinning (102,103). This suggests that many of the vascular changes seen on OCTA are secondary to the optic atrophy rather than underlying mechanism of optic nerve injury (102). Longitudinal studies of glaucomatous eyes and eyes in the acute stages of AION are required to determine if OCTA will be able to detect the underlying ischemic changes that are thought to contribute to these diseases, and whether these changes can predict the disease course and outcome.
Swept source OCT has recently become available, which uses a longer wave-length (1,050 nm) and has faster scan speeds (100,000 scans/s) than spectral domain OCT. Swept source imaging provides improved visualization of the choroid and deeper structures of the optic nerve, such as the lamina cribrosa. As stated earlier, this feature may make swept source OCT particularly useful in the evaluation of ODD. Detailed imaging of the lamina cribrosa also has important ramifications for both glaucoma and papilledema, which are felt to be on the opposite ends of a double-edged sword (104). In the setting of low cerebrospinal fluid pressure, there is a negative translaminar gradient and back-bowing of the lamina cribrosa that has been hypothesized to contribute to glaucomatous damage (105-107). Conversely, raised intracranial pressure causes a forward displacement of the Bruch’s membrane, which presumably reflects vector forces at the level of the lamina cribrosa (51). Swept source OCT provides improved visualization of the lamina cribrosa, and therefore intracranial pressure effects on the lamina cribrosa could be directly measured in future studies. These tools may inform our understanding of why astronauts develop papilledema and other ophthalmic conditions after long-duration space flights, which has been called spaceflight associated neuro-ocular syndrome (SANS) (108).
OCT has been touted to provide a “window to the brain” as a potential biomarker for neurodegenerative conditions. Multiple studies have shown RNFL and GCIP thinning in patients with Alzheimer’s disease compared to age matched controls, which were summarized in a meta-analysis by Coppola and colleagues (109). In addition, similar findings are seen with Parkinson’s disease and frontotemporal dementia (110-112). Abnormalities in the vascular structure of the retina have also been recently found on OCTA in patients with Alzheimer’s disease (113), which supports the hypothesis that vascular abnormalities may be involved in the pathobiology of this disease. While OCT appears to have a promising role in the diagnosis and prognostication of patients suspected of having neurodegenerative conditions, future longitudinal studies will be required to determine its usefulness in clinical practice.
Key points: OCT future directions and new applications
OCT has revolutionized the practice of neuro-ophthalmology by improving our diagnostic and prognostic abilities across multiple diseases. As discussed in this review, OCT is a useful tool for diagnosing and monitoring demyelinating disease, ODD, papilledema, and various other optic neuropathies. It is an integral part of the evaluation for a patient with unexplained vision loss because it can differentiate between optic neuropathy and retinal disease. The applications for OCT are countless and its role is constantly expanding, including its use as a potential biomarker for neurodegenerative conditions. As OCT technology continues to advance, it will undoubtedly augment our ability to diagnose and better understand the pathophysiology of optic nerve diseases in the future.