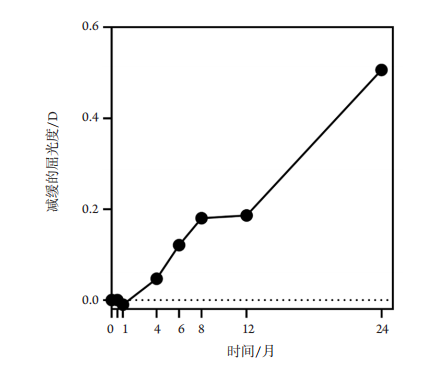
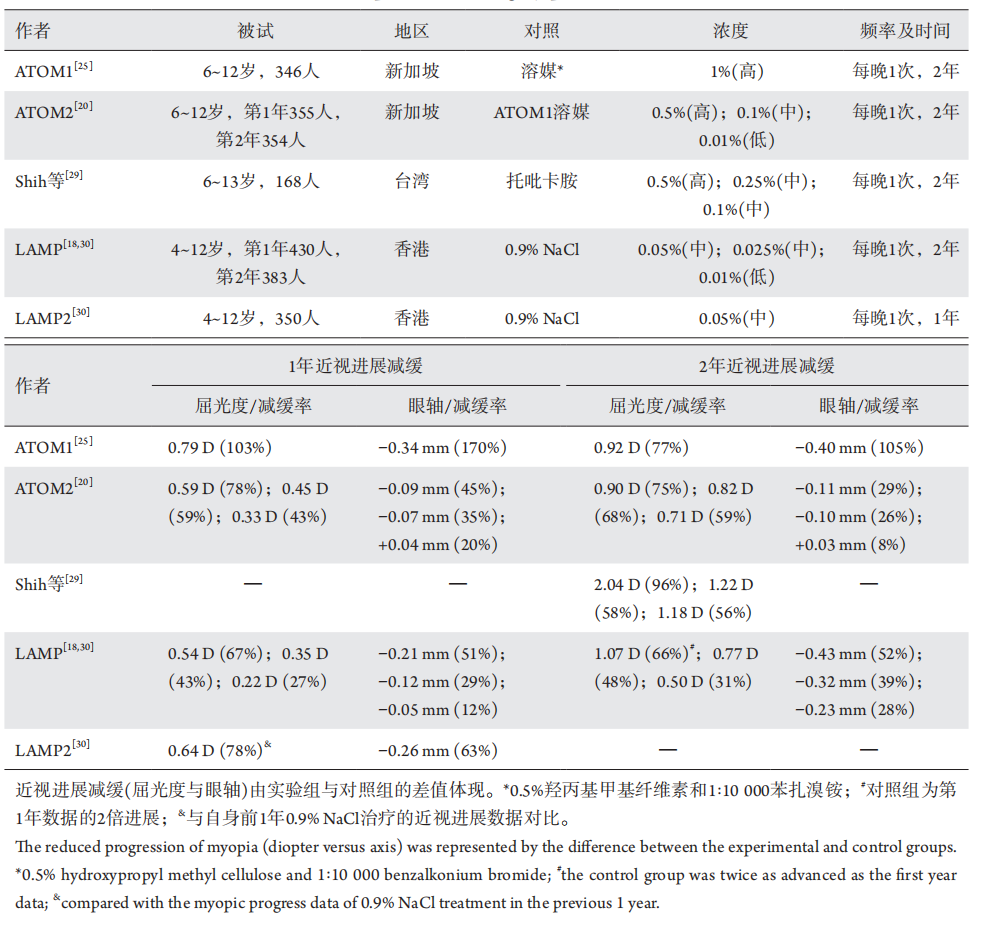
1、Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia
and high myopia and temporal trends from 2000 through 2050[ J].
Ophthalmology, 2016, 123(5): 1036-1042.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia
and high myopia and temporal trends from 2000 through 2050[ J].
Ophthalmology, 2016, 123(5): 1036-1042.
2、Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations
and time trends in the prevalence of childhood myopia, a systematic
review and quantitative meta-analysis: implications for aetiology and
early prevention[ J]. Br J Ophthalmol, 2016, 100(7): 882-890.Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations
and time trends in the prevalence of childhood myopia, a systematic
review and quantitative meta-analysis: implications for aetiology and
early prevention[ J]. Br J Ophthalmol, 2016, 100(7): 882-890.
3、Xie Z, Long Y, Wang J, et al. Prevalence of myopia and associated risk
factors among primary students in Chongqing: multilevel modeling[ J].
BMC Ophthalmol, 2020, 20(1): 146.Xie Z, Long Y, Wang J, et al. Prevalence of myopia and associated risk
factors among primary students in Chongqing: multilevel modeling[ J].
BMC Ophthalmol, 2020, 20(1): 146.
4、Liu S, Ye S, Wang Q, et al. Breastfeeding and myopia: A cross-sectional
study of children aged 6-12 years in Tianjin, China[ J]. Sci Rep, 2018,
8(1): 10025.Liu S, Ye S, Wang Q, et al. Breastfeeding and myopia: A cross-sectional
study of children aged 6-12 years in Tianjin, China[ J]. Sci Rep, 2018,
8(1): 10025.
5、Lyu Y, Zhang H, Gong Y, et al. Prevalence of and factors associated with
myopia in primary school students in the Chaoyang District of Beijing,
China[ J]. Jpn J Ophthalmol, 2015, 59(6): 421-429.Lyu Y, Zhang H, Gong Y, et al. Prevalence of and factors associated with
myopia in primary school students in the Chaoyang District of Beijing,
China[ J]. Jpn J Ophthalmol, 2015, 59(6): 421-429.
6、Wu JF, Bi HS, Wang SM, et al. Refractive error, visual acuity and causes
of vision loss in children in Shandong, China. The Shandong Children
Eye Study[ J]. PLoS One, 2013, 8(12): e82763.Wu JF, Bi HS, Wang SM, et al. Refractive error, visual acuity and causes
of vision loss in children in Shandong, China. The Shandong Children
Eye Study[ J]. PLoS One, 2013, 8(12): e82763.
7、Dolgin E. The myopia boom[ J]. Nature, 2015, 519(7543): 276-278.Dolgin E. The myopia boom[ J]. Nature, 2015, 519(7543): 276-278.
8、Wang J, Li Y, Musch DC, et al. Progression of myopia in school-aged
children after COVID-19 home confinement[ J]. JAMA Ophthalmol,
2021, 139(3): 293-300.Wang J, Li Y, Musch DC, et al. Progression of myopia in school-aged
children after COVID-19 home confinement[ J]. JAMA Ophthalmol,
2021, 139(3): 293-300.
9、Chua SY, Sabanayagam C, Cheung YB, et al. Age of onset of myopia
predicts risk of high myopia in later childhood in myopic Singapore
children[ J]. Ophthalmic Physiol Opt, 2016, 36(4): 388-394.Chua SY, Sabanayagam C, Cheung YB, et al. Age of onset of myopia
predicts risk of high myopia in later childhood in myopic Singapore
children[ J]. Ophthalmic Physiol Opt, 2016, 36(4): 388-394.
10、Tang SM, Chan RY, Bin Lin S, et al. Refractive errors and concomitant
strabismus: a systematic review and meta-analysis[ J]. Sci Rep, 2016,
6: 35177.Tang SM, Chan RY, Bin Lin S, et al. Refractive errors and concomitant
strabismus: a systematic review and meta-analysis[ J]. Sci Rep, 2016,
6: 35177.
11、?azarczyk JB, Urban B, Konarzewska B, et al. The differences in level of
trait anxiety among girls and boys aged 13-17 years with myopia and
emmetropia[ J]. BMC Ophthalmol, 2016, 16(1): 201?azarczyk JB, Urban B, Konarzewska B, et al. The differences in level of
trait anxiety among girls and boys aged 13-17 years with myopia and
emmetropia[ J]. BMC Ophthalmol, 2016, 16(1): 201
12、Modjtahedi BS, Ferris FL 3rd, Hunter DG, et al. Public health burden
and potential interventions for myopia[ J]. Ophthalmology, 2018,
125(5): 628-630.Modjtahedi BS, Ferris FL 3rd, Hunter DG, et al. Public health burden
and potential interventions for myopia[ J]. Ophthalmology, 2018,
125(5): 628-630.
13、Liang YB, Friedman DS, Wong TY, et al. Prevalence and causes of low
vision and blindness in a rural Chinese adult population: the Handan
Eye Study[ J]. Ophthalmology, 2008, 115(11): 1965-1972.Liang YB, Friedman DS, Wong TY, et al. Prevalence and causes of low
vision and blindness in a rural Chinese adult population: the Handan
Eye Study[ J]. Ophthalmology, 2008, 115(11): 1965-1972.
14、Resnikoff S, Jonas JB, Friedman D, et al. Myopia - a 21st century public
health issue[ J]. Invest Ophthalmol Vis Sci, 2019, 60(3): Mi-Mii.Resnikoff S, Jonas JB, Friedman D, et al. Myopia - a 21st century public
health issue[ J]. Invest Ophthalmol Vis Sci, 2019, 60(3): Mi-Mii.
15、Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions
for myopia control in children: a network meta-analysis[ J].
Ophthalmology, 2016, 123(4): 697-708Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions
for myopia control in children: a network meta-analysis[ J].
Ophthalmology, 2016, 123(4): 697-708
16、Wei S, Li SM, An W, et al. Safety and efficacy of low-dose atropine
eyedrops for the treatment of myopia progression in Chinese children:
a randomized clinical trial[ J]. JAMA Ophthalmol, 2020, 138(11):
1178-1184.Wei S, Li SM, An W, et al. Safety and efficacy of low-dose atropine
eyedrops for the treatment of myopia progression in Chinese children:
a randomized clinical trial[ J]. JAMA Ophthalmol, 2020, 138(11):
1178-1184.
17、Fu A, Stapleton F, Wei L, et al. Effect of low-dose atropine on myopia
progression, pupil diameter and accommodative amplitude: low-dose
atropine and myopia progression[ J]. Br J Ophthalmol, 2020, 104(11):
1535-1541.Fu A, Stapleton F, Wei L, et al. Effect of low-dose atropine on myopia
progression, pupil diameter and accommodative amplitude: low-dose
atropine and myopia progression[ J]. Br J Ophthalmol, 2020, 104(11):
1535-1541.
18、Yam JC, Jiang Y, Tang SM, et al. Low-concentration atropine for myopia
progression (LAMP) study: a randomized, double-blinded, placebo�controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in
myopia control[ J]. Ophthalmology, 2019, 126(1): 113-124.Yam JC, Jiang Y, Tang SM, et al. Low-concentration atropine for myopia
progression (LAMP) study: a randomized, double-blinded, placebo�controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in
myopia control[ J]. Ophthalmology, 2019, 126(1): 113-124.
19、Zhao Q, Hao Q. Clinical efficacy of 0.01% atropine in retarding the
progression of myopia in children[ J]. Int Ophthalmol, 2021, 41(3):
1011-1017.Zhao Q, Hao Q. Clinical efficacy of 0.01% atropine in retarding the
progression of myopia in children[ J]. Int Ophthalmol, 2021, 41(3):
1011-1017.
20、Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of
childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses
(atropine for the treatment of myopia 2)[ J]. Ophthalmology, 2012,
119(2): 347-354.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of
childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses
(atropine for the treatment of myopia 2)[ J]. Ophthalmology, 2012,
119(2): 347-354.
21、Hieda O, Hiraoka T, Fujikado T, et al. Efficacy and safety of 0.01%
atropine for prevention of childhood myopia in a 2-year randomized
placebo-controlled study[ J]. Jpn J Ophthalmol, 2021, 65(3): 315-325.Hieda O, Hiraoka T, Fujikado T, et al. Efficacy and safety of 0.01%
atropine for prevention of childhood myopia in a 2-year randomized
placebo-controlled study[ J]. Jpn J Ophthalmol, 2021, 65(3): 315-325.
22、Jethani J. Efficacy of low-concentration atropine (0.01%) eye drops
for prevention of axial myopic progression in premyopes[ J]. Indian J
Ophthalmol, 2022, 70(1): 238-240.Jethani J. Efficacy of low-concentration atropine (0.01%) eye drops
for prevention of axial myopic progression in premyopes[ J]. Indian J
Ophthalmol, 2022, 70(1): 238-240.
23、Chia A, Chua WH, Wen L, et al. Atropine for the treatment of
childhood myopia: changes after stopping atropine 0.01%, 0.1% and
0.5%[ J]. Am J Ophthalmol, 2014, 157(2): 451-457.e1.Chia A, Chua WH, Wen L, et al. Atropine for the treatment of
childhood myopia: changes after stopping atropine 0.01%, 0.1% and
0.5%[ J]. Am J Ophthalmol, 2014, 157(2): 451-457.e1.
24、Tong L, Huang XL, Koh AL, et al. Atropine for the treatment of
childhood myopia: effect on myopia progression after cessation of
atropine[ J]. Ophthalmology, 2009, 116(3): 572-579.Tong L, Huang XL, Koh AL, et al. Atropine for the treatment of
childhood myopia: effect on myopia progression after cessation of
atropine[ J]. Ophthalmology, 2009, 116(3): 572-579.
25、Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment
of childhood myopia[ J]. Ophthalmology, 2006, 113(12): 2285-2291.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment
of childhood myopia[ J]. Ophthalmology, 2006, 113(12): 2285-2291.
26、Diaz-Llopis M, Pinazo-Durán MD. Superdiluted atropine at 0.01%
reduces progression in children and adolescents. A 5 year study of
safety and effectiveness[ J]. Arch Soc Esp Oftalmol (Engl Ed), 2018,
93(4): 182-185.Diaz-Llopis M, Pinazo-Durán MD. Superdiluted atropine at 0.01%
reduces progression in children and adolescents. A 5 year study of
safety and effectiveness[ J]. Arch Soc Esp Oftalmol (Engl Ed), 2018,
93(4): 182-185.
27、Chia A , Lu QS, Tan D. Five-year clinical trial on atropine for
the treatment of myopia 2: myopia control with atropine 0.01%
eyedrops[ J]. Ophthalmology, 2016, 123(2): 391-399.Chia A , Lu QS, Tan D. Five-year clinical trial on atropine for
the treatment of myopia 2: myopia control with atropine 0.01%
eyedrops[ J]. Ophthalmology, 2016, 123(2): 391-399.
28、Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of
atropine in childhood myopia: a meta-analysis[ J]. JAMA Ophthalmol,
2017, 135(6): 624-630.Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of
atropine in childhood myopia: a meta-analysis[ J]. JAMA Ophthalmol,
2017, 135(6): 624-630.
29、Shih YF, Chen CH, Chou AC, et al. Effects of different concentrations
of atropine on controlling myopia in myopic children[ J]. J Ocul
Pharmacol Ther, 1999, 15(1): 85-90.Shih YF, Chen CH, Chou AC, et al. Effects of different concentrations
of atropine on controlling myopia in myopic children[ J]. J Ocul
Pharmacol Ther, 1999, 15(1): 85-90.
30、Yam JC, Li FF, Zhang X, et al. Two-year clinical trial of the low�concentration atropine for myopia progression (LAMP) study: phase 2
report[ J]. Ophthalmology, 2020, 127(7): 910-919.Yam JC, Li FF, Zhang X, et al. Two-year clinical trial of the low�concentration atropine for myopia progression (LAMP) study: phase 2
report[ J]. Ophthalmology, 2020, 127(7): 910-919.
31、Li FF, Zhang Y, Zhang X, et al. Age effect on treatment responses
to 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine
for myopia progression study[ J]. Ophthalmology, 2021, 128(8):
1180-1187.Li FF, Zhang Y, Zhang X, et al. Age effect on treatment responses
to 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine
for myopia progression study[ J]. Ophthalmology, 2021, 128(8):
1180-1187.
32、Pediatric Eye Disease Investigator Group. A randomized trial of
atropine vs. patching for treatment of moderate amblyopia in
children[ J]. Arch Ophthalmol, 2002, 120(3): 268-278.Pediatric Eye Disease Investigator Group. A randomized trial of
atropine vs. patching for treatment of moderate amblyopia in
children[ J]. Arch Ophthalmol, 2002, 120(3): 268-278.
33、Yen MY, Liu JH, Kao SC, et al. Comparison of the effect of atropine
and cyclopentolate on myopia[ J]. Ann Ophthalmol, 1989, 21(5):
180-2, 187.Yen MY, Liu JH, Kao SC, et al. Comparison of the effect of atropine
and cyclopentolate on myopia[ J]. Ann Ophthalmol, 1989, 21(5):
180-2, 187.
34、Foo LL, Htoon H, Farooqui SZ, et al. Part-time use of 1% atropine
eye drops for prevention of myopia progression in children[ J]. Int
Ophthalmol, 2020, 40(7): 1857-1862.Foo LL, Htoon H, Farooqui SZ, et al. Part-time use of 1% atropine
eye drops for prevention of myopia progression in children[ J]. Int
Ophthalmol, 2020, 40(7): 1857-1862.
35、Fang PC, Chung MY, Yu HJ, et al. Prevention of myopia onset with
0.025% atropine in premyopic children[ J]. J Ocul Pharmacol Ther,
2010, 26(4): 341-345.Fang PC, Chung MY, Yu HJ, et al. Prevention of myopia onset with
0.025% atropine in premyopic children[ J]. J Ocul Pharmacol Ther,
2010, 26(4): 341-345.
36、Loh KL, Lu Q, Tan D, et al. Risk factors for progressive myopia in the
atropine therapy for myopia study[ J]. Am J Ophthalmol, 2015, 159(5):
945-949.Loh KL, Lu Q, Tan D, et al. Risk factors for progressive myopia in the
atropine therapy for myopia study[ J]. Am J Ophthalmol, 2015, 159(5):
945-949.
37、Lyu Y, Ji N, Fu AC, et al. Comparison of administration of 0.02%
atropine and orthokeratology for myopia control[ J]. Eye Contact Lens,
2021, 47(2): 81-85.Lyu Y, Ji N, Fu AC, et al. Comparison of administration of 0.02%
atropine and orthokeratology for myopia control[ J]. Eye Contact Lens,
2021, 47(2): 81-85.
38、Ye L, Shi Y, Yin Y, et al. Effects of atropine treatment on choroidal
thickness in myopic children[ J]. Invest Ophthalmol Vis Sci, 2020,
61(14): 15.Ye L, Shi Y, Yin Y, et al. Effects of atropine treatment on choroidal
thickness in myopic children[ J]. Invest Ophthalmol Vis Sci, 2020,
61(14): 15.
39、游巍, 钱峰, 吴诚. 眼部的给药屏障和给药途径[ J]. 国际药学研
究杂志, 2008, 35(4): 284-287.
YOU Wei, QIAN Feng, WU Cheng. Barriers and methods of ocular
drug delivery[ J]. Journal of International Pharmaceutical Research,
2008, 35(4): 284-287.游巍, 钱峰, 吴诚. 眼部的给药屏障和给药途径[ J]. 国际药学研
究杂志, 2008, 35(4): 284-287.
YOU Wei, QIAN Feng, WU Cheng. Barriers and methods of ocular
drug delivery[ J]. Journal of International Pharmaceutical Research,
2008, 35(4): 284-287.
40、Meisner D, Pringle J, Mezei M. Liposomal ophthalmic drug delivery.
III. Phar-macodynamic and biodisposition studies of atropine[ J].
International Journal of Pharma-ceutics, 1989, 55(2): 105-113.Meisner D, Pringle J, Mezei M. Liposomal ophthalmic drug delivery.
III. Phar-macodynamic and biodisposition studies of atropine[ J].
International Journal of Pharma-ceutics, 1989, 55(2): 105-113.
41、Janes RG, Stiles JF. The penetration of C14-labeled atropine into
the eye; a comparison of methods of application[ J]. AMA Arch
Ophthalmol, 1959, 62(1): 69-74.Janes RG, Stiles JF. The penetration of C14-labeled atropine into
the eye; a comparison of methods of application[ J]. AMA Arch
Ophthalmol, 1959, 62(1): 69-74.
42、Mori N, Mochizuki T, Yamazaki F, et al. MALDI imaging mass
spectrometry revealed atropine distribution in the ocular tissues and its
transit from anterior to posterior regions in the whole-eye of rabbit after
topical administration[ J]. PLoS One, 2019, 14(1): e0211376.Mori N, Mochizuki T, Yamazaki F, et al. MALDI imaging mass
spectrometry revealed atropine distribution in the ocular tissues and its
transit from anterior to posterior regions in the whole-eye of rabbit after
topical administration[ J]. PLoS One, 2019, 14(1): e0211376.
43、Salazar M, Patil PN. An explanation for the long duration of mydriatic
effect of atropine in eye[ J]. Invest Ophthalmol, 1976, 15(8): 671-673.Salazar M, Patil PN. An explanation for the long duration of mydriatic
effect of atropine in eye[ J]. Invest Ophthalmol, 1976, 15(8): 671-673.
44、Wang LZ, Syn N, Li S, et al. The penetration and distribution of topical
atropine in animal ocular tissues[ J]. Acta Ophthalmol, 2019, 97(2):
e238-e247.Wang LZ, Syn N, Li S, et al. The penetration and distribution of topical
atropine in animal ocular tissues[ J]. Acta Ophthalmol, 2019, 97(2):
e238-e247.
45、Salazar M, Shimada K, Patil PN. Iris pigmentation and atropine
mydriasis[ J]. J Pharmacol Exp Ther, 1976, 197(1): 79-88.Salazar M, Shimada K, Patil PN. Iris pigmentation and atropine
mydriasis[ J]. J Pharmacol Exp Ther, 1976, 197(1): 79-88.
46、Ji M, Liu H, Ma S, et al. Stable atropine loaded film as a potential ocular
delivery system for treatment of myopia[ J]. Pharm Res, 2021, 38(11):
1931-1946.Ji M, Liu H, Ma S, et al. Stable atropine loaded film as a potential ocular
delivery system for treatment of myopia[ J]. Pharm Res, 2021, 38(11):
1931-1946.
47、Barathi VA, Beuerman RW, Schaeffel F. Effects of unilateral topical
atropine on binocular pupil responses and eye growth in mice[ J].
Vision Res, 2009, 49(3): 383-387.Barathi VA, Beuerman RW, Schaeffel F. Effects of unilateral topical
atropine on binocular pupil responses and eye growth in mice[ J].
Vision Res, 2009, 49(3): 383-387.
48、Li FF, Kam KW, Zhang Y, et al. Differential effects on ocular biometrics
by 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine
for myopia progression study[ J]. Ophthalmology, 2020, 127(12):
1603-1611.Li FF, Kam KW, Zhang Y, et al. Differential effects on ocular biometrics
by 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine
for myopia progression study[ J]. Ophthalmology, 2020, 127(12):
1603-1611.
49、Ye L, Li S, Shi Y, et al. Comparisons of atropine versus cyclopentolate
cycloplegia in myopic children[ J]. Clin Exp Optom, 2021, 104(2):
143-150.Ye L, Li S, Shi Y, et al. Comparisons of atropine versus cyclopentolate
cycloplegia in myopic children[ J]. Clin Exp Optom, 2021, 104(2):
143-150.
50、Chen Z, Huang S, Zhou J, et al. Adjunctive effect of orthokeratology
and low dose atropine on axial elongation in fast-progressing myopic
children-A preliminary retrospective study[ J]. Cont Lens Anterior Eye,
2019, 42(4): 439-442.Chen Z, Huang S, Zhou J, et al. Adjunctive effect of orthokeratology
and low dose atropine on axial elongation in fast-progressing myopic
children-A preliminary retrospective study[ J]. Cont Lens Anterior Eye,
2019, 42(4): 439-442.
51、Ross AE, Bengani LC, Tulsan R, et al. Topical sustained drug delivery
to the retina with a drug-eluting contact lens[ J]. Biomaterials, 2019,
217: 119285.Ross AE, Bengani LC, Tulsan R, et al. Topical sustained drug delivery
to the retina with a drug-eluting contact lens[ J]. Biomaterials, 2019,
217: 119285.
52、Sun HY, Lu WY, You JY, et al. Peripheral Refraction in Myopic
Children with and without Atropine Usage[ J]. J Ophthalmol,
2020, 2020: 4919154.Sun HY, Lu WY, You JY, et al. Peripheral Refraction in Myopic
Children with and without Atropine Usage[ J]. J Ophthalmol,
2020, 2020: 4919154.
53、Gardner DJ, Walline JJ, Mutti DO. Choroidal thickness and peripheral
myopic defocus during orthokeratology[ J]. Optom Vis Sci, 2015,
92(5): 579-588.Gardner DJ, Walline JJ, Mutti DO. Choroidal thickness and peripheral
myopic defocus during orthokeratology[ J]. Optom Vis Sci, 2015,
92(5): 579-588.
54、Sankaridurg P, Bakaraju RC, Naduvilath T, et al. Myopia control with
novel central and peripheral plus contact lenses and extended depth of
focus contact lenses: 2 year results from a randomised clinical trial[ J].
Ophthalmic Physiol Opt, 2019, 39(4): 294-307.Sankaridurg P, Bakaraju RC, Naduvilath T, et al. Myopia control with
novel central and peripheral plus contact lenses and extended depth of
focus contact lenses: 2 year results from a randomised clinical trial[ J].
Ophthalmic Physiol Opt, 2019, 39(4): 294-307.
55、Tigges M, Iuvone PM, Fernandes A, et al. Effects of muscarinic
cholinergic receptor antagonists on postnatal eye growth of rhesus
monkeys[ J]. Optom Vis Sci, 1999, 76(6): 397-407.Tigges M, Iuvone PM, Fernandes A, et al. Effects of muscarinic
cholinergic receptor antagonists on postnatal eye growth of rhesus
monkeys[ J]. Optom Vis Sci, 1999, 76(6): 397-407.
56、Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia
control[ J]. Proc Natl Acad Sci U S A, 2018, 115(30): E7091-E7100.Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia
control[ J]. Proc Natl Acad Sci U S A, 2018, 115(30): E7091-E7100.
57、Greene PR. Mechanical considerations in myopia: relative effects of
accommodation, convergence, intraocular pressure, and the extraocular
muscles[ J]. Am J Optom Physiol Opt, 1980, 57(12): 902-914.Greene PR. Mechanical considerations in myopia: relative effects of
accommodation, convergence, intraocular pressure, and the extraocular
muscles[ J]. Am J Optom Physiol Opt, 1980, 57(12): 902-914.
58、McBrien NA , Moghaddam HO, Reeder AP. Atropine reduces
experimental myopia and eye enlargement via a nonaccommodative
mechanism[ J]. Invest Ophthalmol Vis Sci, 1993, 34(1): 205-215.McBrien NA , Moghaddam HO, Reeder AP. Atropine reduces
experimental myopia and eye enlargement via a nonaccommodative
mechanism[ J]. Invest Ophthalmol Vis Sci, 1993, 34(1): 205-215.
59、Collison DJ, Coleman RA, James RS, et al. Characterization of
muscarinic receptors in human lens cells by pharmacologic and
molecular techniques[ J]. Invest Ophthalmol Vis Sci, 2000, 41(9):
2633-2641.Collison DJ, Coleman RA, James RS, et al. Characterization of
muscarinic receptors in human lens cells by pharmacologic and
molecular techniques[ J]. Invest Ophthalmol Vis Sci, 2000, 41(9):
2633-2641.
60、Gil DW, Krauss HA, Bogardus AM, et al. Muscarinic receptor subtypes
in human iris-ciliary body measured by immunoprecipitation[ J]. Invest
Ophthalmol Vis Sci, 1997, 38(7): 1434-1442.Gil DW, Krauss HA, Bogardus AM, et al. Muscarinic receptor subtypes
in human iris-ciliary body measured by immunoprecipitation[ J]. Invest
Ophthalmol Vis Sci, 1997, 38(7): 1434-1442.
61、Qu J, Zhou X, Xie R, et al. The presence of m1 to m5 receptors in
human sclera: evidence of the sclera as a potential site of action for
muscarinic receptor antagonists[ J]. Curr Eye Res, 2006, 31(7/8):
587-597.Qu J, Zhou X, Xie R, et al. The presence of m1 to m5 receptors in
human sclera: evidence of the sclera as a potential site of action for
muscarinic receptor antagonists[ J]. Curr Eye Res, 2006, 31(7/8):
587-597.
62、Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive
development, dopamine release, and slow retinal potentials in the
chick[ J]. Vis Neurosci, 2000, 17(2): 165-176.Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive
development, dopamine release, and slow retinal potentials in the
chick[ J]. Vis Neurosci, 2000, 17(2): 165-176.
63、Schaeffel F, Troilo D, Wallman J, et al. Developing eyes that lack
accommodation grow to compensate for imposed defocus[ J]. Vis
Neurosci, 1990, 4(2): 177-183.Schaeffel F, Troilo D, Wallman J, et al. Developing eyes that lack
accommodation grow to compensate for imposed defocus[ J]. Vis
Neurosci, 1990, 4(2): 177-183.
64、Schmid KL, Wildsoet CF. Effects on the compensatory responses to
positive and negative lenses of intermittent lens wear and ciliary nerve
section in chicks[ J]. Vision Res, 1996, 36(7): 1023-1036.Schmid KL, Wildsoet CF. Effects on the compensatory responses to
positive and negative lenses of intermittent lens wear and ciliary nerve
section in chicks[ J]. Vision Res, 1996, 36(7): 1023-1036.
65、Fischer AJ, Miethke P, Morgan IG, et al. Cholinergic amacrine cells are
not required for the progression and atropine-mediated suppression of
form-deprivation myopia[ J]. Brain Res, 1998, 794(1): 48-60.Fischer AJ, Miethke P, Morgan IG, et al. Cholinergic amacrine cells are
not required for the progression and atropine-mediated suppression of
form-deprivation myopia[ J]. Brain Res, 1998, 794(1): 48-60.
66、Vessey KA, Cottriall CL, McBrien NA. Muscarinic receptor protein
expression in the ocular tissues of the chick during normal and myopic
eye development[ J]. Brain Res Dev Brain Res, 2002, 135(1-2): 79-86.Vessey KA, Cottriall CL, McBrien NA. Muscarinic receptor protein
expression in the ocular tissues of the chick during normal and myopic
eye development[ J]. Brain Res Dev Brain Res, 2002, 135(1-2): 79-86.
67、Gallego P, Martínez-García C, Pérez-Merino P, et al. Scleral changes
induced by atropine in chicks as an experimental model of myopia[ J].
Ophthalmic Physiol Opt, 2012, 32(6): 478-484.Gallego P, Martínez-García C, Pérez-Merino P, et al. Scleral changes
induced by atropine in chicks as an experimental model of myopia[ J].
Ophthalmic Physiol Opt, 2012, 32(6): 478-484.
68、Barathi VA, Kwan JL, Tan QS, et al. Muscarinic cholinergic receptor
(M2) plays a crucial role in the development of myopia in mice[ J]. Dis
Model Mech, 2013, 6(5): 1146-1158.Barathi VA, Kwan JL, Tan QS, et al. Muscarinic cholinergic receptor
(M2) plays a crucial role in the development of myopia in mice[ J]. Dis
Model Mech, 2013, 6(5): 1146-1158.
69、Liu Q, Wu J, Wang X, et al. Changes in muscarinic acetylcholine
receptor expression in form deprivation myopia in guinea pigs[ J]. Mol
Vis, 2007, 13: 1234-1244.Liu Q, Wu J, Wang X, et al. Changes in muscarinic acetylcholine
receptor expression in form deprivation myopia in guinea pigs[ J]. Mol
Vis, 2007, 13: 1234-1244.
70、Lin HJ, Wan L, Chen WC, et al. Muscarinic acetylcholine receptor 3 is
dominant in myopia progression[ J]. Invest Ophthalmol Vis Sci, 2012,
53(10): 6519-6525.Lin HJ, Wan L, Chen WC, et al. Muscarinic acetylcholine receptor 3 is
dominant in myopia progression[ J]. Invest Ophthalmol Vis Sci, 2012,
53(10): 6519-6525.
71、McBrien NA, Arumugam B, Gentle A, et al. The M4 muscarinic
antagonist MT-3 inhibits myopia in chick: evidence for site of
action[ J]. Ophthalmic Physiol Opt, 2011, 31(5): 529-539.McBrien NA, Arumugam B, Gentle A, et al. The M4 muscarinic
antagonist MT-3 inhibits myopia in chick: evidence for site of
action[ J]. Ophthalmic Physiol Opt, 2011, 31(5): 529-539.
72、Barathi VA, Beuerman RW. Molecular mechanisms of muscarinic
receptors in mouse scleral fibroblasts: Prior to and after induction of
experimental myopia with atropine treatment[ J]. Mol Vis, 2011, 17:
680-692.Barathi VA, Beuerman RW. Molecular mechanisms of muscarinic
receptors in mouse scleral fibroblasts: Prior to and after induction of
experimental myopia with atropine treatment[ J]. Mol Vis, 2011, 17:
680-692.
73、Arumugam B, McBrien NA. Muscarinic antagonist control of myopia:
evidence for M4 and M1 receptor-based pathways in the inhibition
of experimentally-induced axial myopia in the tree shrew[ J]. Invest
Ophthalmol Vis Sci, 2012, 53(9): 5827-5837.Arumugam B, McBrien NA. Muscarinic antagonist control of myopia:
evidence for M4 and M1 receptor-based pathways in the inhibition
of experimentally-induced axial myopia in the tree shrew[ J]. Invest
Ophthalmol Vis Sci, 2012, 53(9): 5827-5837.
74、Barathi VA, Chaurasia SS, Poidinger M, et al. Involvement of GABA
transporters in atropine-treated myopic retina as revealed by iTRAQ
quantitative proteomics[ J]. J Proteome Res, 2014, 13(11): 4647-4658.Barathi VA, Chaurasia SS, Poidinger M, et al. Involvement of GABA
transporters in atropine-treated myopic retina as revealed by iTRAQ
quantitative proteomics[ J]. J Proteome Res, 2014, 13(11): 4647-4658.
75、Liu Y, Wang Y, Lv H, et al. α-adrenergic agonist brimonidine control of
experimentally induced myopia in guinea pigs: A pilot study[ J]. Mol
Vis, 2017, 23: 785-798.Liu Y, Wang Y, Lv H, et al. α-adrenergic agonist brimonidine control of
experimentally induced myopia in guinea pigs: A pilot study[ J]. Mol
Vis, 2017, 23: 785-798.
76、Carr BJ, Mihara K , Ramachandran R , et al. Myopia-inhibiting
concentrations of muscarinic receptor antagonists block activation of
alpha2A-adrenoceptors in vitro[ J]. Invest Ophthalmol Vis Sci, 2018,
59(7): 2778-2791.Carr BJ, Mihara K , Ramachandran R , et al. Myopia-inhibiting
concentrations of muscarinic receptor antagonists block activation of
alpha2A-adrenoceptors in vitro[ J]. Invest Ophthalmol Vis Sci, 2018,
59(7): 2778-2791.
77、Zhou X, Pardue MT, Iuvone PM, et al. Dopamine signaling and myopia
development: What are the key challenges[ J]. Prog Retin Eye Res,
2017, 61: 60-71.Zhou X, Pardue MT, Iuvone PM, et al. Dopamine signaling and myopia
development: What are the key challenges[ J]. Prog Retin Eye Res,
2017, 61: 60-71.
78、L andi s EG, Chrenek M A , Chakrabor t y R , et al . Increased
endogenous dopamine prevents myopia in mice[ J]. Exp Eye Res, 2020,
193: 107956L andi s EG, Chrenek M A , Chakrabor t y R , et al . Increased
endogenous dopamine prevents myopia in mice[ J]. Exp Eye Res, 2020,
193: 107956
79、Huang F, Wang Q, Yan T, et al. The role of the dopamine d2 receptor
in form-deprivation myopia in mice: studies with full and partial d2
receptor agonists and knockouts[ J]. Invest Ophthalmol Vis Sci, 2020,
61(6): 47.Huang F, Wang Q, Yan T, et al. The role of the dopamine d2 receptor
in form-deprivation myopia in mice: studies with full and partial d2
receptor agonists and knockouts[ J]. Invest Ophthalmol Vis Sci, 2020,
61(6): 47.
80、Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light
intensity in a school-based cluster randomized trial[ J]. Ophthalmology,
2018, 125(8): 1239-1250.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light
intensity in a school-based cluster randomized trial[ J]. Ophthalmology,
2018, 125(8): 1239-1250.
81、Prepas SB. Light, literacy and the absence of ultraviolet radiation in the
development of myopia[ J]. Med Hypotheses, 2008, 70(3): 635-637.Prepas SB. Light, literacy and the absence of ultraviolet radiation in the
development of myopia[ J]. Med Hypotheses, 2008, 70(3): 635-637.
82、Lin HJ, Wei CC, Chang CY, et al. Role of chronic inflammation in
myopia progression: clinical evidence and experimental validation[ J].
EBioMedicine, 2016, 10: 269-281Lin HJ, Wei CC, Chang CY, et al. Role of chronic inflammation in
myopia progression: clinical evidence and experimental validation[ J].
EBioMedicine, 2016, 10: 269-281
83、Zhang S, Zhang G, Zhou X, et al. Changes in choroidal thickness and
choroidal blood perfusion in guinea pig myopia[ J]. Invest Ophthalmol
Vis Sci, 2019, 60(8): 3074-3083.Zhang S, Zhang G, Zhou X, et al. Changes in choroidal thickness and
choroidal blood perfusion in guinea pig myopia[ J]. Invest Ophthalmol
Vis Sci, 2019, 60(8): 3074-3083.
84、Zhou X, Zhang S, Zhang G, et al. Increased choroidal blood perfusion
can inhibit form deprivation myopia in guinea pigs[ J]. Invest
Ophthalmol Vis Sci, 2020, 61(13): 25.Zhou X, Zhang S, Zhang G, et al. Increased choroidal blood perfusion
can inhibit form deprivation myopia in guinea pigs[ J]. Invest
Ophthalmol Vis Sci, 2020, 61(13): 25.
85、Bullimore MA, Berntsen DA. Low-dose atropine for myopia control:
considering all the data[ J]. JAMA Ophthalmol, 2018, 136(3): 303.Bullimore MA, Berntsen DA. Low-dose atropine for myopia control:
considering all the data[ J]. JAMA Ophthalmol, 2018, 136(3): 303.
86、Shih YF, Hsiao CK, Chen CJ, et al. An intervention trial on efficacy of
atropine and multi-focal glasses in controlling myopic progression[ J].
Acta Ophthalmol Scand, 2001, 79(3): 233-236.Shih YF, Hsiao CK, Chen CJ, et al. An intervention trial on efficacy of
atropine and multi-focal glasses in controlling myopic progression[ J].
Acta Ophthalmol Scand, 2001, 79(3): 233-236.
87、Hao Q, Zhao Q. Changes in subfoveal choroidal thickness in myopic
children with 0.01% atropine, orthokeratology, or their combination[ J].
Int Ophthalmol, 2021, 41(9): 2963-2971.Hao Q, Zhao Q. Changes in subfoveal choroidal thickness in myopic
children with 0.01% atropine, orthokeratology, or their combination[ J].
Int Ophthalmol, 2021, 41(9): 2963-2971.