1、Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010[J]. Br J Ophthalmol, 2012, 96(5): 614-618.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010[J]. Br J Ophthalmol, 2012, 96(5): 614-618.
2、Tan DT, Dart JK, Holland EJ, et al. Corneal transplantation[J]. Lancet, 2012, 379(9827): 1749-1761.Tan DT, Dart JK, Holland EJ, et al. Corneal transplantation[J]. Lancet, 2012, 379(9827): 1749-1761.
3、Burton MJ, Ramke J, Marques AP, et al. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020[J]. Lancet Glob Health, 2021, 9(4): e489-e551.Burton MJ, Ramke J, Marques AP, et al. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020[J]. Lancet Glob Health, 2021, 9(4): e489-e551.
4、Bothun ED, Cleveland J, Lynn MJ, et al. One-year strabismus outcomes in the Infant Aphakia Treatment Study[J]. Ophthalmology, 2013, 120(6): 1227-1231.Bothun ED, Cleveland J, Lynn MJ, et al. One-year strabismus outcomes in the Infant Aphakia Treatment Study[J]. Ophthalmology, 2013, 120(6): 1227-1231.
5、Infant Aphakia Treatment Study Group, Lambert SR, Buckley EG, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year[J]. Arch Ophthalmol, 2010, 128(7): 810-818.Infant Aphakia Treatment Study Group, Lambert SR, Buckley EG, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year[J]. Arch Ophthalmol, 2010, 128(7): 810-818.
6、Jin ZB, Gao ML, Deng WL, et al. Stemming retinal regeneration with pluripotent stem cells[J]. Prog Retin Eye Res, 2019, 69: 38-56.Jin ZB, Gao ML, Deng WL, et al. Stemming retinal regeneration with pluripotent stem cells[J]. Prog Retin Eye Res, 2019, 69: 38-56.
7、Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration[J]. Neuron, 2012, 75(1): 26-39.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration[J]. Neuron, 2012, 75(1): 26-39.
8、Davis BM, Crawley L, Pahlitzsch M, et al. Glaucoma: the retina and beyond[J]. Acta Neuropathol, 2016, 132(6): 807-826.Davis BM, Crawley L, Pahlitzsch M, et al. Glaucoma: the retina and beyond[J]. Acta Neuropathol, 2016, 132(6): 807-826.
9、Wong TY, Cheung CM, Larsen M, et al. Diabetic retinopathy[J]. Nat Rev Dis Primers, 2016, 2: 16012.Wong TY, Cheung CM, Larsen M, et al. Diabetic retinopathy[J]. Nat Rev Dis Primers, 2016, 2: 16012.
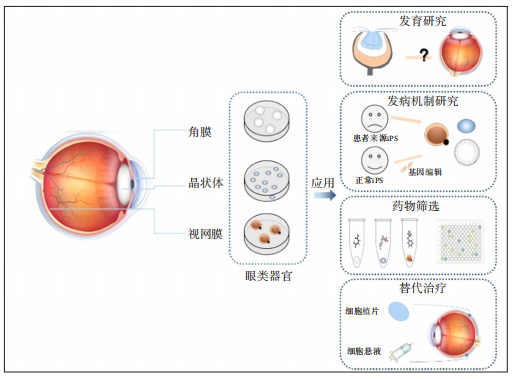
10、Manafi N, Shokri F, Achberger K, et al. Organoids and organ chips in ophthalmology[J]. Ocul Surf, 2021, 19: 1-15.Manafi N, Shokri F, Achberger K, et al. Organoids and organ chips in ophthalmology[J]. Ocul Surf, 2021, 19: 1-15.
11、Stern JH, Tian Y, Funderburgh J, et al. Regenerating Eye Tissues to Preserve and Restore Vision[J]. Cell Stem Cell, 2018, 22(6): 834-849.Stern JH, Tian Y, Funderburgh J, et al. Regenerating Eye Tissues to Preserve and Restore Vision[J]. Cell Stem Cell, 2018, 22(6): 834-849.
12、Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies[J]. Science, 2014, 345(6194): 1247125.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies[J]. Science, 2014, 345(6194): 1247125.
13、Holloway EM, Capeling MM, Spence JR. Biologically inspired approaches to enhance human organoid complexity[J]. Development, 2019, 146(8): dev166173.Holloway EM, Capeling MM, Spence JR. Biologically inspired approaches to enhance human organoid complexity[J]. Development, 2019, 146(8): dev166173.
14、Muthuswamy SK. Bringing together the organoid field: from early beginnings to the road ahead[J]. Development, 2017, 144(6): 963-967.Muthuswamy SK. Bringing together the organoid field: from early beginnings to the road ahead[J]. Development, 2017, 144(6): 963-967.
15、Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations[J]. Exp Cell Res, 1975, 94(1): 70-78.Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations[J]. Exp Cell Res, 1975, 94(1): 70-78.
16、Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals[J]. Cell Stem Cell, 2008, 3(5): 519-532.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals[J]. Cell Stem Cell, 2008, 3(5): 519-532.
17、Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium[J]. Proc Natl Acad Sci U S A, 2009, 106(31): 12771-12775.Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium[J]. Proc Natl Acad Sci U S A, 2009, 106(31): 12771-12775.
18、Sakaguchi H, Kadoshima T, Soen M, et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue[J]. Nat Commun, 2015, 6: 8896.Sakaguchi H, Kadoshima T, Soen M, et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue[J]. Nat Commun, 2015, 6: 8896.
19、Clevers H. Modeling Development and Disease with Organoids[J]. Cell, 2016, 165(7): 1586-1597.Clevers H. Modeling Development and Disease with Organoids[J]. Cell, 2016, 165(7): 1586-1597.
20、Artegiani B, Clevers H. Use and application of 3D-organoid technology[J]. Hum Mol Genet, 2018, 27(R2): R99-R107.Artegiani B, Clevers H. Use and application of 3D-organoid technology[J]. Hum Mol Genet, 2018, 27(R2): R99-R107.
21、Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research[J]. Nat Rev Genet, 2018, 19(11): 671-687.Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research[J]. Nat Rev Genet, 2018, 19(11): 671-687.
22、Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases[J]. Bioessays, 2004, 26(4): 374-386.Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases[J]. Bioessays, 2004, 26(4): 374-386.
23、Graw J. Eye development[J]. Curr Top Dev Biol, 2010, 90: 343-386.Graw J. Eye development[J]. Curr Top Dev Biol, 2010, 90: 343-386.
24、Jackson CJ, Myklebust Erno IT, Ringstad H, et al. Simple limbal epithelial transplantation: current status and future perspectives[J]. Stem Cells Transl Med, 2020, 9(3): 316-327.Jackson CJ, Myklebust Erno IT, Ringstad H, et al. Simple limbal epithelial transplantation: current status and future perspectives[J]. Stem Cells Transl Med, 2020, 9(3): 316-327.
25、Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface[J]. J Cell Biol, 1999, 145(4): 769-782.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface[J]. J Cell Biol, 1999, 145(4): 769-782.
26、Bremond-Gignac D, Copin H, Benkhalifa M. Corneal epithelial stem cells for corneal injury[J]. Expert Opin Biol Ther, 2018, 18(9): 997-1003.Bremond-Gignac D, Copin H, Benkhalifa M. Corneal epithelial stem cells for corneal injury[J]. Expert Opin Biol Ther, 2018, 18(9): 997-1003.
27、Nosrati H, Alizadeh Z, Nosrati A, et al. Stem cell-based therapeutic strategies for corneal epithelium regeneration[J]. Tissue Cell, 2021, 68: 101470.Nosrati H, Alizadeh Z, Nosrati A, et al. Stem cell-based therapeutic strategies for corneal epithelium regeneration[J]. Tissue Cell, 2021, 68: 101470.
28、Nurkovic JS, Vojinovic R, Dolicanin Z. Corneal stem cells as a source of regenerative cell-based therapy[J]. Stem Cells Int, 2020, 2020: 8813447.Nurkovic JS, Vojinovic R, Dolicanin Z. Corneal stem cells as a source of regenerative cell-based therapy[J]. Stem Cells Int, 2020, 2020: 8813447.
29、Ouyang H, Xue Y, Lin Y, et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis[J]. Nature, 2014, 511(7509): 358-361.Ouyang H, Xue Y, Lin Y, et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis[J]. Nature, 2014, 511(7509): 358-361.
30、Hayashi R, Ishikawa Y, Sasamoto Y, et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function[J]. Nature, 2016, 531(7594): 376-380.Hayashi R, Ishikawa Y, Sasamoto Y, et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function[J]. Nature, 2016, 531(7594): 376-380.
31、Shibata S, Hayashi R, Okubo T, et al. Selective laminin-directed differentiation of human induced pluripotent stem cells into distinct ocular lineages[J]. Cell Rep, 2018, 25(6): 1668-1679.e1665.Shibata S, Hayashi R, Okubo T, et al. Selective laminin-directed differentiation of human induced pluripotent stem cells into distinct ocular lineages[J]. Cell Rep, 2018, 25(6): 1668-1679.e1665.
32、Minami Y, Sugihara H, Oono S. Reconstruction of cornea in three-dimensional collagen gel matrix culture[J]. Invest Ophthalmol Vis Sci, 1993, 34(7): 2316-2324.Minami Y, Sugihara H, Oono S. Reconstruction of cornea in three-dimensional collagen gel matrix culture[J]. Invest Ophthalmol Vis Sci, 1993, 34(7): 2316-2324.
33、Germain L, Auger FA, Grandbois E, et al. Reconstructed human cornea produced in vitro by tissue engineering[J]. Pathobiology, 1999, 67(3): 140-147.Germain L, Auger FA, Grandbois E, et al. Reconstructed human cornea produced in vitro by tissue engineering[J]. Pathobiology, 1999, 67(3): 140-147.
34、Griffith M, Osborne R, Munger R, et al. Functional human corneal equivalents constructed from cell lines[J]. Science, 1999, 286(5447): 2169-2172.Griffith M, Osborne R, Munger R, et al. Functional human corneal equivalents constructed from cell lines[J]. Science, 1999, 286(5447): 2169-2172.
35、Foster JW, Wahlin K, Adams SM, et al. Cornea organoids from human induced pluripotent stem cells[J]. Sci Rep, 2017, 7: 41286.Foster JW, Wahlin K, Adams SM, et al. Cornea organoids from human induced pluripotent stem cells[J]. Sci Rep, 2017, 7: 41286.
36、Susaimanickam PJ, Maddileti S, Pulimamidi VK, et al. Generating minicorneal organoids from human induced pluripotent stem cells[J]. Development, 2017, 144(13): 2338-2351.Susaimanickam PJ, Maddileti S, Pulimamidi VK, et al. Generating minicorneal organoids from human induced pluripotent stem cells[J]. Development, 2017, 144(13): 2338-2351.
37、Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration[J]. N Engl J Med, 2010, 363(2): 147-155.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration[J]. N Engl J Med, 2010, 363(2): 147-155.
38、Kolli S, Ahmad S, Lako M, et al. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency[J]. Stem Cells, 2010, 28(3): 597-610.Kolli S, Ahmad S, Lako M, et al. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency[J]. Stem Cells, 2010, 28(3): 597-610.
39、Zakaria N, Possemiers T, Dhubhghaill SN, et al. Results of a phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation[J]. J Transl Med, 2014, 12: 58.Zakaria N, Possemiers T, Dhubhghaill SN, et al. Results of a phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation[J]. J Transl Med, 2014, 12: 58.
40、Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells[J]. N Engl J Med, 2000, 343(2): 86-93.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells[J]. N Engl J Med, 2000, 343(2): 86-93.
41、Lopez-Garcia JS, Rivas Jara L, Garcia-Lozano I, et al. Histopathologic limbus evolution after alkaline burns[J]. Cornea, 2007, 26(9): 1043-1048.Lopez-Garcia JS, Rivas Jara L, Garcia-Lozano I, et al. Histopathologic limbus evolution after alkaline burns[J]. Cornea, 2007, 26(9): 1043-1048.
42、Ting DSJ, Peh GSL, Adnan K, et al. Translational and regulatory challenges of corneal endothelial cell therapy: a global perspective[J/OL]. Tissue Eng Part B Rev, 2021, Epub ahead of print.Ting DSJ, Peh GSL, Adnan K, et al. Translational and regulatory challenges of corneal endothelial cell therapy: a global perspective[J/OL]. Tissue Eng Part B Rev, 2021, Epub ahead of print.
43、Bennet D, Estlack Z, Reid T, et al. A microengineered human corneal epithelium-on-a-chip for eye drops mass transport evaluation[J]. Lab Chip, 2018, 18(11): 1539-1551.Bennet D, Estlack Z, Reid T, et al. A microengineered human corneal epithelium-on-a-chip for eye drops mass transport evaluation[J]. Lab Chip, 2018, 18(11): 1539-1551.
44、Seo J, Byun WY, Alisafaei F, et al. Multiscale reverse engineering of the human ocular surface[J]. Nat Med, 2019, 25(8): 1310-1318.Seo J, Byun WY, Alisafaei F, et al. Multiscale reverse engineering of the human ocular surface[J]. Nat Med, 2019, 25(8): 1310-1318.
45、Cvekl A, Ashery-Padan R. The cellular and molecular mechanisms of vertebrate lens development[J]. Development, 2014, 141(23): 4432-4447.Cvekl A, Ashery-Padan R. The cellular and molecular mechanisms of vertebrate lens development[J]. Development, 2014, 141(23): 4432-4447.
46、Ogino H, Ochi H, Reza HM, et al. Transcription factors involved in lens development from the preplacodal ectoderm[J]. Dev Biol, 2012, 363(2): 333-347.Ogino H, Ochi H, Reza HM, et al. Transcription factors involved in lens development from the preplacodal ectoderm[J]. Dev Biol, 2012, 363(2): 333-347.
47、Huang FL, Russell P, Kuwabara T. Fine structure of lentoid bodies derived from normal and cataractous mouse lenses[J]. Exp Eye Res, 1980, 31(5): 535-541.Huang FL, Russell P, Kuwabara T. Fine structure of lentoid bodies derived from normal and cataractous mouse lenses[J]. Exp Eye Res, 1980, 31(5): 535-541.
48、Yang C, Yang Y, Brennan L, et al. Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions[J]. FASEB J, 2010, 24(9): 3274-3283.Yang C, Yang Y, Brennan L, et al. Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions[J]. FASEB J, 2010, 24(9): 3274-3283.
49、Fu Q, Qin Z, Jin X, et al. Generation of functional lentoid bodies from human induced pluripotent stem cells derived from urinary cells[J]. Invest Ophthalmol Vis Sci, 2017, 58(1): 517-527.Fu Q, Qin Z, Jin X, et al. Generation of functional lentoid bodies from human induced pluripotent stem cells derived from urinary cells[J]. Invest Ophthalmol Vis Sci, 2017, 58(1): 517-527.
50、Murphy P, Kabir MH, Srivastava T, et al. Light-focusing human micro-lenses generated from pluripotent stem cells model lens development and drug-induced cataract in vitro[J]. Development, 2018, 145(1): dev155838.Murphy P, Kabir MH, Srivastava T, et al. Light-focusing human micro-lenses generated from pluripotent stem cells model lens development and drug-induced cataract in vitro[J]. Development, 2018, 145(1): dev155838.
51、Han C, Li J, Wang C, et al. Wnt5a contributes to the differentiation of human embryonic stem cells into lentoid bodies through the noncanonical Wnt/JNK signaling pathway[J]. Invest Ophthalmol Vis Sci, 2018, 59(8): 3449-3460.Han C, Li J, Wang C, et al. Wnt5a contributes to the differentiation of human embryonic stem cells into lentoid bodies through the noncanonical Wnt/JNK signaling pathway[J]. Invest Ophthalmol Vis Sci, 2018, 59(8): 3449-3460.
52、Fu Q, Qin Z, Zhang L, et al. A new long noncoding RNA ALB regulates autophagy by enhancing the transformation of LC3BI to LC3BII during human lens development[J]. Mol Ther Nucleic Acids, 2017, 9: 207-217.Fu Q, Qin Z, Zhang L, et al. A new long noncoding RNA ALB regulates autophagy by enhancing the transformation of LC3BI to LC3BII during human lens development[J]. Mol Ther Nucleic Acids, 2017, 9: 207-217.
53、Qin Z, Zhang L, Lyu D, et al. Opacification of lentoid bodies derived from human induced pluripotent stem cells is accelerated by hydrogen peroxide and involves protein aggregation[J]. J Cell Physiol, 2019, 234(12): 23750-23762.Qin Z, Zhang L, Lyu D, et al. Opacification of lentoid bodies derived from human induced pluripotent stem cells is accelerated by hydrogen peroxide and involves protein aggregation[J]. J Cell Physiol, 2019, 234(12): 23750-23762.
54、Lin H, Ouyang H, Zhu J, et al. Lens regeneration using endogenous stem cells with gain of visual function[J]. Nature, 2016, 531(7594): 323-328.Lin H, Ouyang H, Zhu J, et al. Lens regeneration using endogenous stem cells with gain of visual function[J]. Nature, 2016, 531(7594): 323-328.
55、Marquardt T. Transcriptional control of neuronal diversification in the retina[J]. Prog Retin Eye Res, 2003, 22(5): 567-577.Marquardt T. Transcriptional control of neuronal diversification in the retina[J]. Prog Retin Eye Res, 2003, 22(5): 567-577.
56、Heavner W, Pevny L. Eye development and retinogenesis[J]. Cold Spring Harb Perspect Biol, 2012, 4(12): a008391.Heavner W, Pevny L. Eye development and retinogenesis[J]. Cold Spring Harb Perspect Biol, 2012, 4(12): a008391.
57、Jin K, Xiang M. Transitional Progenitors during Vertebrate Retinogenesis[J]. Mol Neurobiol, 2017, 54(5): 3565-3576.Jin K, Xiang M. Transitional Progenitors during Vertebrate Retinogenesis[J]. Mol Neurobiol, 2017, 54(5): 3565-3576.
58、Wan J, Goldman D. Retina regeneration in zebrafish[J]. Curr Opin Genet Dev, 2016, 40: 41-47.Wan J, Goldman D. Retina regeneration in zebrafish[J]. Curr Opin Genet Dev, 2016, 40: 41-47.
59、Goldman D. Muller glial cell reprogramming and retina regeneration[J]. Nat Rev Neurosci, 2014, 15(7): 431-442.Goldman D. Muller glial cell reprogramming and retina regeneration[J]. Nat Rev Neurosci, 2014, 15(7): 431-442.
60、Eiraku M, Sasai Y. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues[J]. Nat Protoc, 2011, 7(1): 69-79.Eiraku M, Sasai Y. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues[J]. Nat Protoc, 2011, 7(1): 69-79.
61、Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture[J]. Nature, 2011, 472(7341): 51-56.Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture[J]. Nature, 2011, 472(7341): 51-56.
62、Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs[J]. Cell Stem Cell, 2012, 10(6): 771-785.Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs[J]. Cell Stem Cell, 2012, 10(6): 771-785.
63、Zhong X, Gutierrez C, Xue T, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs[J]. Nat Commun, 2014, 5: 4047.Zhong X, Gutierrez C, Xue T, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs[J]. Nat Commun, 2014, 5: 4047.
64、Lowe A, Harris R, Bhansali P, et al. Intercellular adhesion-dependent cell survival and rock-regulated actomyosin-driven forces mediate self-formation of a retinal organoid[J]. Stem Cell Reports, 2016, 6(5): 743-756.Lowe A, Harris R, Bhansali P, et al. Intercellular adhesion-dependent cell survival and rock-regulated actomyosin-driven forces mediate self-formation of a retinal organoid[J]. Stem Cell Reports, 2016, 6(5): 743-756.
65、Wahlin KJ, Maruotti JA, Sripathi SR, et al. Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells[J]. Sci Rep, 2017, 7(1): 766.Wahlin KJ, Maruotti JA, Sripathi SR, et al. Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells[J]. Sci Rep, 2017, 7(1): 766.
66、Kuwahara A, Ozone C, Nakano T, et al. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue[J]. Nat Commun, 2015, 6: 6286.Kuwahara A, Ozone C, Nakano T, et al. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue[J]. Nat Commun, 2015, 6: 6286.
67、Regent F, Chen HY, Kelley RA, et al. A simple and efficient method for generating human retinal organoids[J]. Mol Vis, 2020, 26: 97-105.Regent F, Chen HY, Kelley RA, et al. A simple and efficient method for generating human retinal organoids[J]. Mol Vis, 2020, 26: 97-105.
68、Reichman S, Slembrouck A, Gagliardi G, et al. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in Xeno-free and feeder-free conditions[J]. Stem Cells, 2017, 35(5): 1176-1188.Reichman S, Slembrouck A, Gagliardi G, et al. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in Xeno-free and feeder-free conditions[J]. Stem Cells, 2017, 35(5): 1176-1188.
69、Capowski EE, Samimi K, Mayerl SJ, et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines[J]. Development, 2019, 146(1): dev171686.Capowski EE, Samimi K, Mayerl SJ, et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines[J]. Development, 2019, 146(1): dev171686.
70、Kim S, Lowe A, Dharmat R, et al. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids[J]. Proc Natl Acad Sci U S A, 2019, 116(22): 10824-10833.Kim S, Lowe A, Dharmat R, et al. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids[J]. Proc Natl Acad Sci U S A, 2019, 116(22): 10824-10833.
71、Cowan CS, Renner M, De Gennaro M, et al. Cell types of the human retina and its organoids at single-cell resolution[J]. Cell, 2020, 182(6): 1623-1640 e1634.Cowan CS, Renner M, De Gennaro M, et al. Cell types of the human retina and its organoids at single-cell resolution[J]. Cell, 2020, 182(6): 1623-1640 e1634.
72、Achberger K, Probst C, Haderspeck J, et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform[J]. Elife, 2019, 8: e46188.Achberger K, Probst C, Haderspeck J, et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform[J]. Elife, 2019, 8: e46188.
73、Volkner M, Zschatzsch M, Rostovskaya M, et al. Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis[J]. Stem Cell Reports, 2016, 6(4): 525-538.Volkner M, Zschatzsch M, Rostovskaya M, et al. Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis[J]. Stem Cell Reports, 2016, 6(4): 525-538.
74、Takata N, Abbey D, Fiore L, et al. An eye organoid approach identifies Six3 suppression of R-spondin 2 as a critical step in mouse neuroretina differentiation[J]. Cell Rep, 2017, 21(6): 1534-1549.Takata N, Abbey D, Fiore L, et al. An eye organoid approach identifies Six3 suppression of R-spondin 2 as a critical step in mouse neuroretina differentiation[J]. Cell Rep, 2017, 21(6): 1534-1549.
75、Capowski EE, Simonett JM, Clark EM, et al. Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation[J]. Hum Mol Genet, 2014, 23(23): 6332-6344.Capowski EE, Simonett JM, Clark EM, et al. Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation[J]. Hum Mol Genet, 2014, 23(23): 6332-6344.
76、Li G, Gao G, Wang P, et al. Generation and characterization of induced pluripotent stem cells and retinal organoids from a Leber's congenital amaurosis patient with novel RPE65 mutations[J]. Front Mol Neurosci, 2019, 12: 212.Li G, Gao G, Wang P, et al. Generation and characterization of induced pluripotent stem cells and retinal organoids from a Leber's congenital amaurosis patient with novel RPE65 mutations[J]. Front Mol Neurosci, 2019, 12: 212.
77、Quinn PM, Buck TM, Mulder AA, et al. Human iPSC-derived retinas recapitulate the fetal CRB1 CRB2 complex formation and demonstrate that photoreceptors and muller glia are targets of AAV5[J]. Stem Cell Reports, 2019, 12(5): 906-919.Quinn PM, Buck TM, Mulder AA, et al. Human iPSC-derived retinas recapitulate the fetal CRB1 CRB2 complex formation and demonstrate that photoreceptors and muller glia are targets of AAV5[J]. Stem Cell Reports, 2019, 12(5): 906-919.
78、Gao ML, Lei XL, Han F, et al. Patient-specific retinal organoids recapitulate disease features of late-onset retinitis pigmentosa[J]. Front Cell Dev Biol, 2020, 8: 128.Gao ML, Lei XL, Han F, et al. Patient-specific retinal organoids recapitulate disease features of late-onset retinitis pigmentosa[J]. Front Cell Dev Biol, 2020, 8: 128.
79、Saengwimol D, Rojanaporn D, Chaitankar V, et al. A three-dimensional organoid model recapitulates tumorigenic aspects and drug responses of advanced human retinoblastoma[J]. Sci Rep, 2018, 8(1): 15664.Saengwimol D, Rojanaporn D, Chaitankar V, et al. A three-dimensional organoid model recapitulates tumorigenic aspects and drug responses of advanced human retinoblastoma[J]. Sci Rep, 2018, 8(1): 15664.
80、Ito SI, Onishi A, Takahashi M. Chemically-induced photoreceptor degeneration and protection in mouse iPSC-derived three-dimensional retinal organoids[J]. Stem Cell Res, 2017, 24: 94-101.Ito SI, Onishi A, Takahashi M. Chemically-induced photoreceptor degeneration and protection in mouse iPSC-derived three-dimensional retinal organoids[J]. Stem Cell Res, 2017, 24: 94-101.
81、Lakowski J, Welby E, Budinger D, et al. Isolation of Human photoreceptor precursors via a cell surface marker panel from stem cell-derived retinal organoids and fetal retinae[J]. Stem Cells, 2018, 36(5): 709-722.Lakowski J, Welby E, Budinger D, et al. Isolation of Human photoreceptor precursors via a cell surface marker panel from stem cell-derived retinal organoids and fetal retinae[J]. Stem Cells, 2018, 36(5): 709-722.
82、Garita-Hernandez M, Lampic M, Chaffiol A, et al. Restoration of visual function by transplantation of optogenetically engineered photoreceptors[J]. Nat Commun, 2019, 10(1): 4524.Garita-Hernandez M, Lampic M, Chaffiol A, et al. Restoration of visual function by transplantation of optogenetically engineered photoreceptors[J]. Nat Commun, 2019, 10(1): 4524.
83、Zou T, Gao L, Zeng Y, et al. Organoid-derived C-Kit(+)/SSEA4(-) human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents[J]. Nat Commun, 2019, 10(1): 1205.Zou T, Gao L, Zeng Y, et al. Organoid-derived C-Kit(+)/SSEA4(-) human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents[J]. Nat Commun, 2019, 10(1): 1205.
84、Hirayama M, Ogawa M, Oshima M, et al. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ[J]. Nat Commun, 2013, 4: 2497.Hirayama M, Ogawa M, Oshima M, et al. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ[J]. Nat Commun, 2013, 4: 2497.
85、Spaniol K, Metzger M, Roth M, et al. Engineering of a secretory active three-dimensional lacrimal gland construct on the basis of decellularized lacrimal gland tissue[J]. Tissue Eng Part A, 2015, 21(19/20): 2605-2617.Spaniol K, Metzger M, Roth M, et al. Engineering of a secretory active three-dimensional lacrimal gland construct on the basis of decellularized lacrimal gland tissue[J]. Tissue Eng Part A, 2015, 21(19/20): 2605-2617.
86、Lin H, Sun G, He H, et al. Three-dimensional culture of functional adult rabbit lacrimal gland epithelial cells on decellularized scaffold[J]. Tissue Eng Part A, 2016, 22(1/2): 65-74.Lin H, Sun G, He H, et al. Three-dimensional culture of functional adult rabbit lacrimal gland epithelial cells on decellularized scaffold[J]. Tissue Eng Part A, 2016, 22(1/2): 65-74.
87、Gromova A, Voronov DA, Yoshida M, et al. Lacrimal gland repair using progenitor cells[J]. Stem Cells Transl Med, 2017, 6(1): 88-98.Gromova A, Voronov DA, Yoshida M, et al. Lacrimal gland repair using progenitor cells[J]. Stem Cells Transl Med, 2017, 6(1): 88-98.
88、Hu Q, Friedrich AM, Johnson LV, et al. Memory in induced pluripotent stem cells: reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation[J]. Stem Cells, 2010, 28(11): 1981-1991.Hu Q, Friedrich AM, Johnson LV, et al. Memory in induced pluripotent stem cells: reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation[J]. Stem Cells, 2010, 28(11): 1981-1991.
89、Wang L, Hiler D, Xu B, et al. Retinal cell type DNA methylation and histone modifications predict reprogramming efficiency and retinogenesis in 3D organoid cultures[J]. Cell Rep, 2018, 22(10): 2601-2614.Wang L, Hiler D, Xu B, et al. Retinal cell type DNA methylation and histone modifications predict reprogramming efficiency and retinogenesis in 3D organoid cultures[J]. Cell Rep, 2018, 22(10): 2601-2614.
90、Hiler D, Chen X, Hazen J, et al. Quantification of retinogenesis in 3D cultures reveals epigenetic memory and higher efficiency in iPSCs derived from rod photoreceptors[J]. Cell Stem Cell, 2015, 17(1): 101-115.Hiler D, Chen X, Hazen J, et al. Quantification of retinogenesis in 3D cultures reveals epigenetic memory and higher efficiency in iPSCs derived from rod photoreceptors[J]. Cell Stem Cell, 2015, 17(1): 101-115.