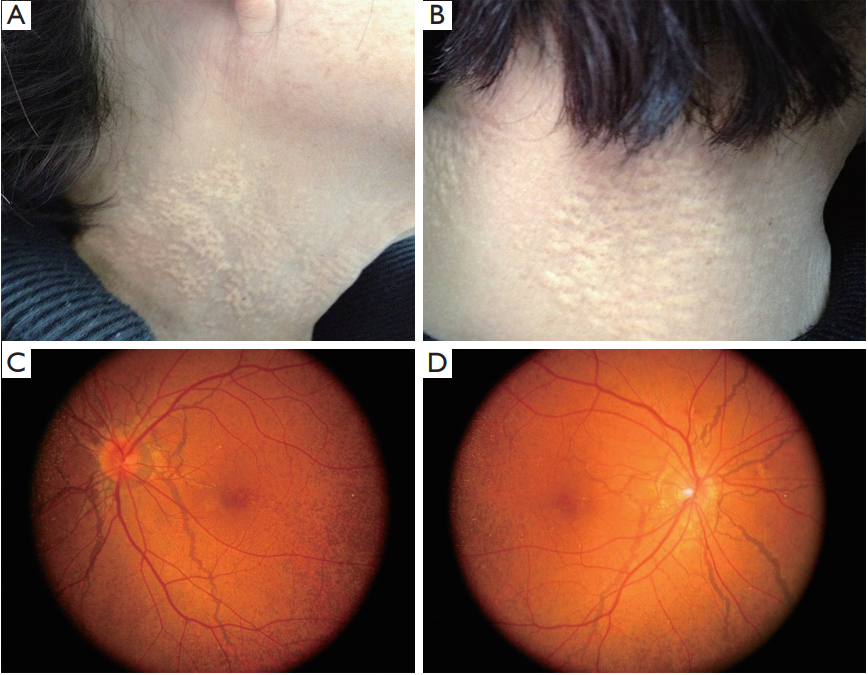
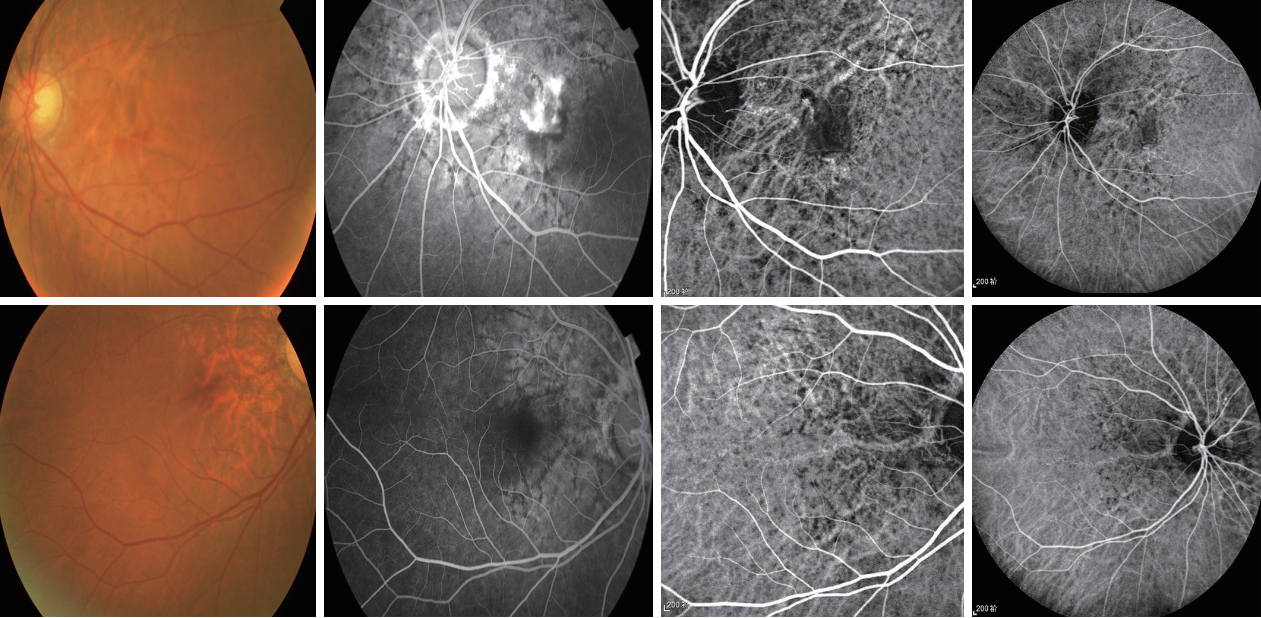
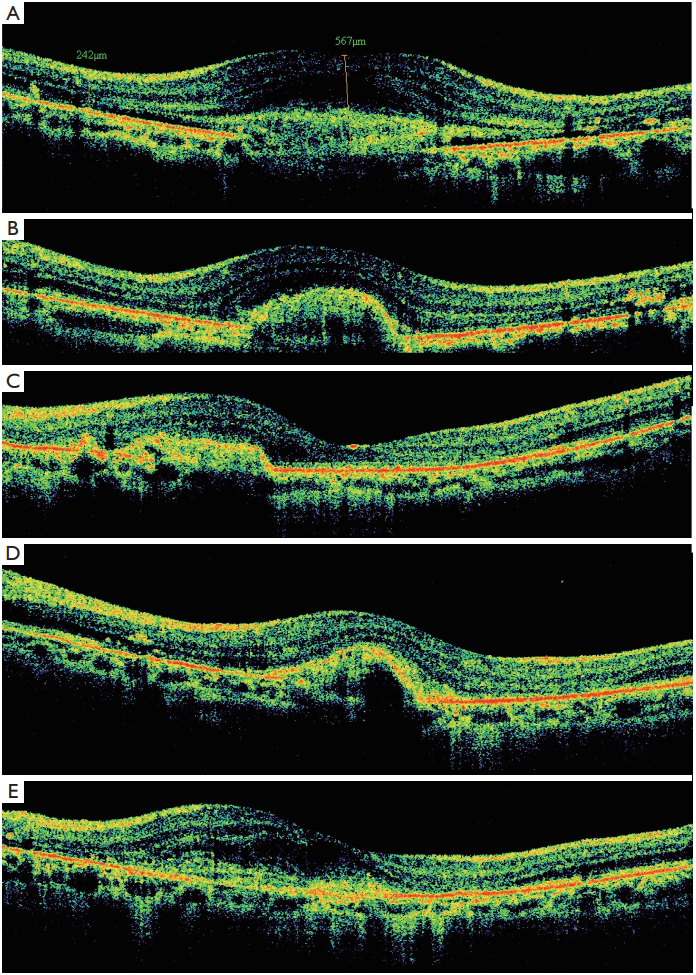
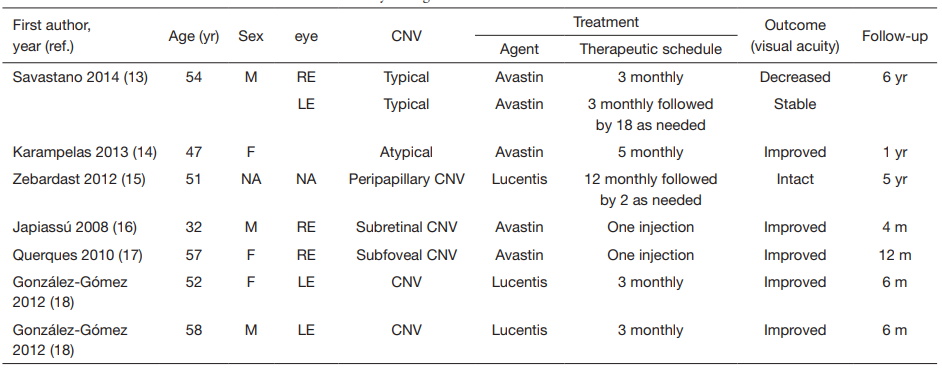
1、Finger RP, Charbel Issa P, Ladewig MS, et al. Pseudoxanthoma elasticum: genetics, clinical
manifestations and therapeutic approaches. Surv Ophthalmol 2009;54:272-85
2、Chassaing N, Martin L, Calvas P, et al. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet
2005;42:881-92
3、Le Saux O, Urban Z, Tschuch C, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 2000;25:223-7.
4、Struk%20B%2C%20Cai%20L%2C%20Z%C3%A4ch%20S%2C%20et%20al.%20Mutations%20of%20the%20gene%20encoding%20the%20transmembrane%20transporter%20protein%20ABC-C6%20cause%20pseudoxanthoma%20elasticum.%20J%20Mol%20Med%20(Berl)%20%0A2000%3B78%3A282-6.
5、Schoenberger SD, Agarwal A. Geographic chorioretinal atrophy in pseudoxanthoma elasticum. Am J Ophthalmol 2013;156:715-23.
6、Georgalas I, Papaconstantinou D, Koutsandrea C, et al. Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clin Risk Manag 2009;5:81-9.
7、Zarbock R, Hendig D, Szliska C, et al. Vascular endothelial growth factor gene polymorphisms as prognostic markers for ocular manifestations in pseudoxanthoma elasticum. Hum Mol Genet 2009;18:3344-51
8、Verbraak FD. Antivascular endothelial growth factor treatment in pseudoxanthoma elasticum patients. Dev Ophthalmol 2010;46:96-106.
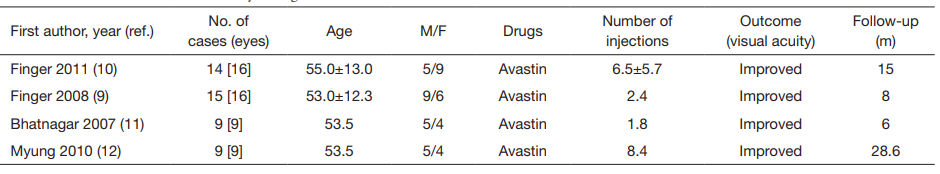
9、Finger RP, Charbel Issa P, Ladewig M, et al. Intravitreal bevacizumab for choroidal neovascularisation associated with pseudoxanthoma elasticum. Br J Ophthalmol
2008;92:483-7.
10、Finger RP, Charbel Issa P, Schmitz-Valckenberg S, et al. Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina 2011;31:1268-78.
11、Bhatnagar P, Freund KB, Spaide RF, et al. Intravitreal bevacizumab for the management of choroidal neovascularization in pseudoxanthoma elasticum. Retina 2007;27:897-902.
12、Myung JS, Bhatnagar P, Spaide RF, et al. Longtermoutcomes of intravitreal antivascular endothelial growth factor therapy for the management of choroidal neovascularization in pseudoxanthoma elasticum. Retina 2010;30:748-55.
13、Savastano MC, Minnella AM, Zinzanella G, et al. Successful long-term management of choroidal neovascularization secondary to angioid streaks in a patient with pseudoxanthoma elasticum: a case report. J Med Case Rep 2014;8:458.
14、Karampelas M, Soumplis V, Karagiannis D, et al. An atypical case of choroidal neovascularization associated with pseudoxanthoma elasticum treated with intravitreal
bevacizumab: a case report. BMC Res Notes 2013;6:530.
15、Zebardast N, Adelman RA. Intravitreal ranibizumab for treatment of choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum: five-year
follow-up. Semin Ophthalmol 2012;27:61-4.
16、Japiass%C3%BA%20RM%2C%20Japiass%C3%BA%20MA%2C%20Pecego%20MG.%20Intravitreal%20bevacizumab%20in%20choroidal%20neovascularization%20secondary%20to%20Gr%C3%B6nblad-Strandberg%20syndrome%3A%20case%20report.%20Arq%20Bras%20%0AOftalmol%202008%3B71%3A427-9.
17、Querques G, Bux AV, Prascina F, et al. Intravitreal avastin for choroidal neovascularization associated with stargardtlikeretinal abnormalities in pseudoxanthoma elasticum. Middle East Afr J Ophthalmol 2010;17:387-9.
18、González-Gómez A, Morillo MJ, González-Escobar AB, et al. Choroidal neovascularization secondary to pseudoxanthoma elasticum treated with ranibizumab: a report of 2 cases. Arch Soc Esp Oftalmol 2012;87:153-6.
19、Lee JM, Nam WH, Kim HK. Photodynamic therapy with verteporfin for choroidal neovascularization in patients with angioid streaks. Korean J Ophthalmol 2007;21:142-5.
20、Jurklies B, Bornfeld N, Schilling H. Photodynamic therapy using verteporfin for choroidal neovascularization associated with angioid streaks--long-term effects. Ophthalmic Res 2006;38:209-17.
21、Arias L, Pujol O, Rubio M, et al. Long-term results of photodynamic therapy for the treatment of choroidal neovascularization secondary to angioid streaks. Graefes Arch Clin Exp Ophthalmol 2006;244:753-7.
22、Pece A, Avanza P, Galli L, et al. Laser photocoagulation of choroidal neovascularization in angioid streaks. Retina 1997;17:12-6.