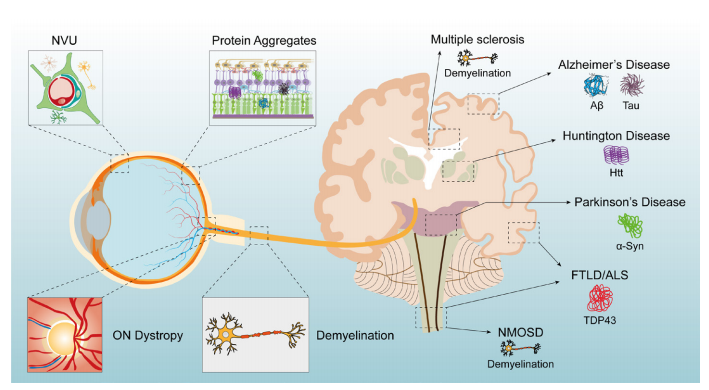
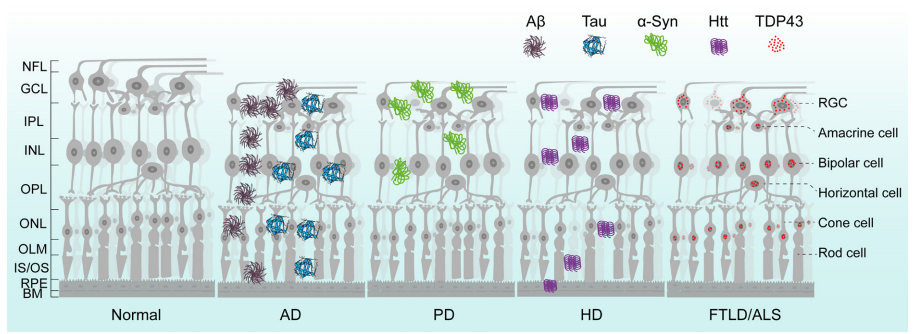
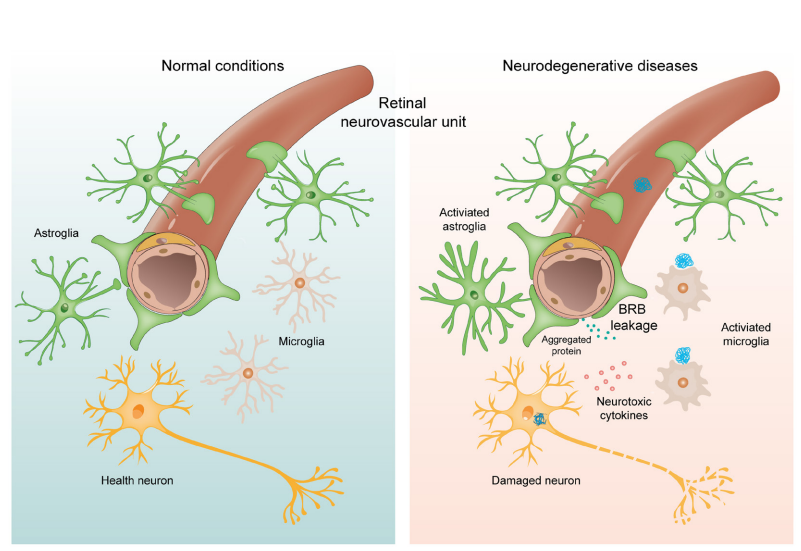
1、Hou Y, Dan X , Babbar M, et al. A geing as a risk factor for
neurodegenerative disease[ J]. Nat Rev Neurol, 2019, 15(10): 565-581.Hou Y, Dan X , Babbar M, et al. A geing as a risk factor for
neurodegenerative disease[ J]. Nat Rev Neurol, 2019, 15(10): 565-581.
2、Golriz Khatami S, Mubeen S, Hofmann-Apitius M. Data science
in neurodegenerative disease: its capabilities, limitations, and
perspectives[ J]. Curr Opin Neurol, 2020, 33(2): 249-254Golriz Khatami S, Mubeen S, Hofmann-Apitius M. Data science
in neurodegenerative disease: its capabilities, limitations, and
perspectives[ J]. Curr Opin Neurol, 2020, 33(2): 249-254
3、Peng C, Trojanowski JQ, Lee VM. Protein transmission in
neurodegenerative disease[ J]. Nat Rev Neurol, 2020, 16(4): 199-212.Peng C, Trojanowski JQ, Lee VM. Protein transmission in
neurodegenerative disease[ J]. Nat Rev Neurol, 2020, 16(4): 199-212.
4、Kovacs GG. Molecular pathology of neurodegenerative diseases:
principles and practice[ J]. J Clin Pathol, 2019, 72(11): 725-735.Kovacs GG. Molecular pathology of neurodegenerative diseases:
principles and practice[ J]. J Clin Pathol, 2019, 72(11): 725-735.
5、Dugger BN, Dickson DW. Pathology of neurodegenerative diseases[ J].
Cold Spring Harb Perspect Biol, 2017, 9(7): a028035.Dugger BN, Dickson DW. Pathology of neurodegenerative diseases[ J].
Cold Spring Harb Perspect Biol, 2017, 9(7): a028035.
6、Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and
epidemiology of the major neurodegenerative diseases[ J]. Cold Spring
Harb Perspect Biol, 2018, 10(4): a033118.Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and
epidemiology of the major neurodegenerative diseases[ J]. Cold Spring
Harb Perspect Biol, 2018, 10(4): a033118.
7、Breijyeh Z, Karaman R. Comprehensive review on alzheimer's disease:
causes and treatment[ J]. Molecules, 2020, 25(24): 5789.Breijyeh Z, Karaman R. Comprehensive review on alzheimer's disease:
causes and treatment[ J]. Molecules, 2020, 25(24): 5789.
8、Jankovic J, Tan EK . Parkinson's disease: etiopathogenesis and
treatment[ J]. J Neurol Neurosurg Psychiatry, 2020, 91(8): 795-808.Jankovic J, Tan EK . Parkinson's disease: etiopathogenesis and
treatment[ J]. J Neurol Neurosurg Psychiatry, 2020, 91(8): 795-808.
9、McColgan P, Tabrizi SJ. Huntington's disease: a clinical review[ J]. Eur J
Neurol, 2018, 25(1): 24-34.McColgan P, Tabrizi SJ. Huntington's disease: a clinical review[ J]. Eur J
Neurol, 2018, 25(1): 24-34.
10、Younes K, Miller BL. Frontotemporal dementia: neuropathology,
genetics, neuroimaging, and treatments[ J]. Psychiatr Clin North Am,
2020, 43(2): 331-344.Younes K, Miller BL. Frontotemporal dementia: neuropathology,
genetics, neuroimaging, and treatments[ J]. Psychiatr Clin North Am,
2020, 43(2): 331-344.
11、Sullivan PM, Zhou X, Robins AM, et al. The ALS/FTLD associated
protein C9orf72 associates with SMCR8 and WDR41 to regulate the
autophagy-lysosome pathway[ J]. Acta Neuropathol Commun, 2016,
4(1): 51.Sullivan PM, Zhou X, Robins AM, et al. The ALS/FTLD associated
protein C9orf72 associates with SMCR8 and WDR41 to regulate the
autophagy-lysosome pathway[ J]. Acta Neuropathol Commun, 2016,
4(1): 51.
12、Zhou X, Sun L, Bracko O, et al. Impaired prosaposin lysosomal
trafficking in frontotemporal lobar degeneration due to progranulin
mutations[ J]. Nat Commun, 2017, 8: 15277.Zhou X, Sun L, Bracko O, et al. Impaired prosaposin lysosomal
trafficking in frontotemporal lobar degeneration due to progranulin
mutations[ J]. Nat Commun, 2017, 8: 15277.
13、Kovacs GG, Botond G, Budka H. Protein coding of neurodegenerative
dementias: the neuropathological basis of biomarker diagnostics[ J].
Acta Neuropathol, 2010, 119(4): 389-408.Kovacs GG, Botond G, Budka H. Protein coding of neurodegenerative
dementias: the neuropathological basis of biomarker diagnostics[ J].
Acta Neuropathol, 2010, 119(4): 389-408.
14、Alzforum. a catalog of the therapeutics currently or previously tested
as treatment for Alzheimer's disease and related disorders [EB/OL].
[2022-05-29]. https://www.alzforum.org/therapeutics.Alzforum. a catalog of the therapeutics currently or previously tested
as treatment for Alzheimer's disease and related disorders [EB/OL].
[2022-05-29]. https://www.alzforum.org/therapeutics.
15、Liu KY, Howard R. Can we learn lessons from the FDA's approval of
aducanumab?[ J]. Nat Rev Neurol, 2021, 17(11): 715-722.Liu KY, Howard R. Can we learn lessons from the FDA's approval of
aducanumab?[ J]. Nat Rev Neurol, 2021, 17(11): 715-722.
16、Lauwers E, Lalli G, Brandner S, et al. Potential human transmission of
amyloid β pathology: surveillance and risks[ J]. Lancet Neurol, 2020,
19(10): 872-878.Lauwers E, Lalli G, Brandner S, et al. Potential human transmission of
amyloid β pathology: surveillance and risks[ J]. Lancet Neurol, 2020,
19(10): 872-878.
17、Chiu K, Chan TF, Wu A, et al. Neurodegeneration of the retina in
mouse models of Alzheimer's disease: what can we learn from the
retina?[ J]. AGE, 2012, 34(3): 633-649.Chiu K, Chan TF, Wu A, et al. Neurodegeneration of the retina in
mouse models of Alzheimer's disease: what can we learn from the
retina?[ J]. AGE, 2012, 34(3): 633-649.
18、Mirzaei N, Shi H, Oviatt M, et al. Alzheimer's retinopathy: seeing
disease in the eyes[ J]. Front Neurosci, 2020, 14: 921.Mirzaei N, Shi H, Oviatt M, et al. Alzheimer's retinopathy: seeing
disease in the eyes[ J]. Front Neurosci, 2020, 14: 921.
19、Ge YJ, Xu W, Ou YN, et al. Retinal biomarkers in Alzheimer's disease
and mild cognitive impairment: a systematic review and meta-analysis[ J]. Ageing Res Rev, 2021, 69: 101361.Ge YJ, Xu W, Ou YN, et al. Retinal biomarkers in Alzheimer's disease
and mild cognitive impairment: a systematic review and meta-analysis[ J]. Ageing Res Rev, 2021, 69: 101361.
20、Berisha F, Feke GT, Trempe CL, et al. Retinal abnormalities in early
Alzheimer's disease[ J]. Invest Ophthalmol Vis Sci, 2007, 48(5): 2285-
2289.Berisha F, Feke GT, Trempe CL, et al. Retinal abnormalities in early
Alzheimer's disease[ J]. Invest Ophthalmol Vis Sci, 2007, 48(5): 2285-
2289.
21、Donix M, Wittig D, Hermann W, et al. Relation of retinal and
hippocampal thickness in patients with amnestic mild cognitive
impairment and healthy controls[ J]. Brain Behav, 2021, 11(5): e02035.Donix M, Wittig D, Hermann W, et al. Relation of retinal and
hippocampal thickness in patients with amnestic mild cognitive
impairment and healthy controls[ J]. Brain Behav, 2021, 11(5): e02035.
22、Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration:
lessons from the Alzheimer's amyloid beta-peptide[ J]. Nat Rev Mol
Cell Biol, 2007, 8(2): 101-112.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration:
lessons from the Alzheimer's amyloid beta-peptide[ J]. Nat Rev Mol
Cell Biol, 2007, 8(2): 101-112.
23、Ohno-Matsui K. Parallel findings in age-related macular degeneration
and Alzheimer's disease[ J]. Prog Retin Eye Res, 2011, 30(4): 217-238.Ohno-Matsui K. Parallel findings in age-related macular degeneration
and Alzheimer's disease[ J]. Prog Retin Eye Res, 2011, 30(4): 217-238.
24、Shah TM, Gupta SM, Chatterjee P, et al. Beta-amyloid sequelae in the
eye: a critical review on its diagnostic significance and clinical relevance
in Alzheimer's disease. Mol Psychiatry, 2017, 22(3): 353-363.Shah TM, Gupta SM, Chatterjee P, et al. Beta-amyloid sequelae in the
eye: a critical review on its diagnostic significance and clinical relevance
in Alzheimer's disease. Mol Psychiatry, 2017, 22(3): 353-363.
25、Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of
amyloid plaques in retinas from Alzheimer's patients and noninvasive in
vivo optical imaging of retinal plaques in a mouse model. Neuroimage, 2011, 54(Suppl 1): S204-S217.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of
amyloid plaques in retinas from Alzheimer's patients and noninvasive in
vivo optical imaging of retinal plaques in a mouse model. Neuroimage, 2011, 54(Suppl 1): S204-S217.
26、Perez SE, Lumayag S, Kovacs B, et al. Beta-amyloid deposition and
functional impairment in the retina of the APPswe/PS1DeltaE9
transgenic mouse model of Alzheimer's disease. Invest Ophthalmol Vis
Sci, 2009, 50(2): 793-800.Perez SE, Lumayag S, Kovacs B, et al. Beta-amyloid deposition and
functional impairment in the retina of the APPswe/PS1DeltaE9
transgenic mouse model of Alzheimer's disease. Invest Ophthalmol Vis
Sci, 2009, 50(2): 793-800.
27、Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, et al. Characterization
of amyloid deposition in the APPswe/PS1dE9 mouse model of
Alzheimer disease. Neurobiol Dis, 2006, 24(3): 516-524.Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, et al. Characterization
of amyloid deposition in the APPswe/PS1dE9 mouse model of
Alzheimer disease. Neurobiol Dis, 2006, 24(3): 516-524.
28、Hart NJ, Koronyo Y, Black KL, et al. Ocular indicators of Alzheimer's:
exploring disease in the retina. Acta Neuropathol, 2016, 132(6): 767-
787.Hart NJ, Koronyo Y, Black KL, et al. Ocular indicators of Alzheimer's:
exploring disease in the retina. Acta Neuropathol, 2016, 132(6): 767-
787.
29、Lynn SA, Johnston DA, Scott JA, et al. Oligomeric Aβ1-42 induces
an AMD-like phenotype and accumulates in lysosomes to impair RPE
function. Cells, 2021, 10(2): 413.Lynn SA, Johnston DA, Scott JA, et al. Oligomeric Aβ1-42 induces
an AMD-like phenotype and accumulates in lysosomes to impair RPE
function. Cells, 2021, 10(2): 413.
30、Dong ZZ, Li J, Gan YF, et al. Amyloid beta deposition related retinal
pigment epithelium cell impairment and subretinal microglia activation
in aged APPswePS1 transgenic mice. Int J Ophthalmol, 2018, 11(5):
747-755.Dong ZZ, Li J, Gan YF, et al. Amyloid beta deposition related retinal
pigment epithelium cell impairment and subretinal microglia activation
in aged APPswePS1 transgenic mice. Int J Ophthalmol, 2018, 11(5):
747-755.
31、Zhao T, Gao J, van J, et al. Age-related increases in amyloid beta and
membrane attack complex: evidence of inflammasome activation in the
rodent eye. J Neuroinflammation, 2015, 12: 121.Zhao T, Gao J, van J, et al. Age-related increases in amyloid beta and
membrane attack complex: evidence of inflammasome activation in the
rodent eye. J Neuroinflammation, 2015, 12: 121.
32、Dutescu RM, Li QX, Crowston J, et al. Amyloid precursor protein
processing and retinal pathology in mouse models of Alzheimer's
disease. Graefes Arch Clin Exp Ophthalmol, 2009, 247(9):1213-1221.Dutescu RM, Li QX, Crowston J, et al. Amyloid precursor protein
processing and retinal pathology in mouse models of Alzheimer's
disease. Graefes Arch Clin Exp Ophthalmol, 2009, 247(9):1213-1221.
33、Gupta VK, Chitranshi N, Gupta VB, et al. Amyloid β accumulation
and inner retinal degenerative changes in Alzheimer's disease transgenic
mouse. Neurosci Lett, 2016, 623: 52-56.Gupta VK, Chitranshi N, Gupta VB, et al. Amyloid β accumulation
and inner retinal degenerative changes in Alzheimer's disease transgenic
mouse. Neurosci Lett, 2016, 623: 52-56.
34、Ning A, Cui J, To E, et al. Amyloid-beta deposits lead to retinal
degeneration in a mouse model of Alzheimer disease[ J]. Invest
Ophthalmol Vis Sci, 2008, 49(11): 5136-5143Ning A, Cui J, To E, et al. Amyloid-beta deposits lead to retinal
degeneration in a mouse model of Alzheimer disease[ J]. Invest
Ophthalmol Vis Sci, 2008, 49(11): 5136-5143
35、Thurgur H, Pinteaux E. Microglia in the Neurovascular Unit: Blood�Brain Barrier-microglia Interactions After Central Nervous System
Disorders[ J]. Neuroscience, 2019, 405: 55-67Thurgur H, Pinteaux E. Microglia in the Neurovascular Unit: Blood�Brain Barrier-microglia Interactions After Central Nervous System
Disorders[ J]. Neuroscience, 2019, 405: 55-67
36、Gendron TF, Petrucelli L. The role of tau in neurodegeneration[ J]. Mol
Neurodegener, 2009, 4: 13.Gendron TF, Petrucelli L. The role of tau in neurodegeneration[ J]. Mol
Neurodegener, 2009, 4: 13.
37、Hernández F, Ferrer I, Pérez M, et al. Tau aggregation[ J]. Neuroscience,
2022: S0306-4522(22)00220.Hernández F, Ferrer I, Pérez M, et al. Tau aggregation[ J]. Neuroscience,
2022: S0306-4522(22)00220.
38、Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration
in Alzheimer's disease and related disorders[ J]. Nat Rev Neurosci,
2007, 8(9): 663-672Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration
in Alzheimer's disease and related disorders[ J]. Nat Rev Neurosci,
2007, 8(9): 663-672
39、Mammadova N, Summers CM, Kokemuller RD, et al. Accelerated
accumulation of retinal α- synuclein( pSer 129 ) and tau ,
neuroinflammation, and autophagic dysregulation in a seeded mouse
model of Parkinson's disease[ J]. Neurobiol Dis, 2019, 121: 1-16.Mammadova N, Summers CM, Kokemuller RD, et al. Accelerated
accumulation of retinal α- synuclein( pSer 129 ) and tau ,
neuroinflammation, and autophagic dysregulation in a seeded mouse
model of Parkinson's disease[ J]. Neurobiol Dis, 2019, 121: 1-16.
40、Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF
measures, and cognition in Alzheimer's disease[ J]. Sci Transl Med,
2016, 8(338): 338ra66.Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF
measures, and cognition in Alzheimer's disease[ J]. Sci Transl Med,
2016, 8(338): 338ra66.
41、Harrison IF, Whitaker R, Bertelli PM, et al. Optic nerve thinning and
neurosensory retinal degeneration in the rTg4510 mouse model of
frontotemporal dementia[ J]. Acta Neuropathol Commun, 2019, 7(1):
4.Harrison IF, Whitaker R, Bertelli PM, et al. Optic nerve thinning and
neurosensory retinal degeneration in the rTg4510 mouse model of
frontotemporal dementia[ J]. Acta Neuropathol Commun, 2019, 7(1):
4.
42、Sch?n C, Hoffmann NA, Ochs SM, et al. Long-term in vivo imaging of
fibrillar tau in the retina of P301S transgenic mice[ J]. PLoS One, 2012,
7(12): e53547.Sch?n C, Hoffmann NA, Ochs SM, et al. Long-term in vivo imaging of
fibrillar tau in the retina of P301S transgenic mice[ J]. PLoS One, 2012,
7(12): e53547.
43、Xia F, Ha Y, Shi S, et al. Early alterations of neurovascular unit in the
retina in mouse models of tauopathy[ J]. Acta Neuropathol Commun,
2021, 9(1): 51.Xia F, Ha Y, Shi S, et al. Early alterations of neurovascular unit in the
retina in mouse models of tauopathy[ J]. Acta Neuropathol Commun,
2021, 9(1): 51.
44、Mullard A. Failure of first anti-tau antibody in Alzheimer disease
highlights risks of history repeating[ J]. Nat Rev Drug Discov, 2021,
20(1): 3-5.Mullard A. Failure of first anti-tau antibody in Alzheimer disease
highlights risks of history repeating[ J]. Nat Rev Drug Discov, 2021,
20(1): 3-5.
45、Ross OA, Braithwaite AT, Skipper LM, et al. Genomic investigation
of alpha-synuclein multiplication and Parkinsonism[ J]. Ann Neurol,
2008, 63(6): 743-750.Ross OA, Braithwaite AT, Skipper LM, et al. Genomic investigation
of alpha-synuclein multiplication and Parkinsonism[ J]. Ann Neurol,
2008, 63(6): 743-750.
46、Issa AR, Sun J, Petitgas C, et al. The lysosomal membrane protein
LAMP2A promotes autophagic flux and prevents SNCA-induced
Parkinson disease-like symptoms in the Drosophila brain[ J].
Autophagy, 2018, 14(11): 1898-1910.Issa AR, Sun J, Petitgas C, et al. The lysosomal membrane protein
LAMP2A promotes autophagic flux and prevents SNCA-induced
Parkinson disease-like symptoms in the Drosophila brain[ J].
Autophagy, 2018, 14(11): 1898-1910.
47、Volpicelli-Daley LA, Gamble KL, Schultheiss CE, et al. Formation
of α-synuclein Lewy neurite-like aggregates in axons impedes the
transport of distinct endosomes[ J]. Mol Biol Cell, 2014, 25(25): 4010-
4023.Volpicelli-Daley LA, Gamble KL, Schultheiss CE, et al. Formation
of α-synuclein Lewy neurite-like aggregates in axons impedes the
transport of distinct endosomes[ J]. Mol Biol Cell, 2014, 25(25): 4010-
4023.
48、Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: Pathology,
mitochondrial dysfunction and neuroinflammation in Parkinson's
disease[ J]. Neurobiol Dis, 2018, 109(Pt B): 249-257.Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: Pathology,
mitochondrial dysfunction and neuroinflammation in Parkinson's
disease[ J]. Neurobiol Dis, 2018, 109(Pt B): 249-257.
49、Mohana Devi S, Mahalaxmi I, Aswathy NP, et al. Does retina play a role
in Parkinson's Disease?[ J]. Acta Neurol Belg, 2020, 120(2): 257-265.Mohana Devi S, Mahalaxmi I, Aswathy NP, et al. Does retina play a role
in Parkinson's Disease?[ J]. Acta Neurol Belg, 2020, 120(2): 257-265.
50、Ortu?o-Lizarán I, Beach TG, Serrano GE, et al. Phosphorylated
α-synuclein in the retina is a biomarker of Parkinson's disease
pathology severity[ J]. Mov Disord, 2018, 33(8): 1315-1324.Ortu?o-Lizarán I, Beach TG, Serrano GE, et al. Phosphorylated
α-synuclein in the retina is a biomarker of Parkinson's disease
pathology severity[ J]. Mov Disord, 2018, 33(8): 1315-1324.
51、Bodis-Wollner I, Kozlowski PB, Glazman S, et al. α-synuclein in the
inner retina in parkinson disease[ J]. Ann Neurol, 2014, 75(6): 964-
966.Bodis-Wollner I, Kozlowski PB, Glazman S, et al. α-synuclein in the
inner retina in parkinson disease[ J]. Ann Neurol, 2014, 75(6): 964-
966.
52、Veys L, Vandenabeele M, Ortu?o-Lizarán I, et al. Retinal α-synuclein
deposits in Parkinson's disease patients and animal models[ J]. Acta
Neuropathol, 2019, 137(3): 379-395.Veys L, Vandenabeele M, Ortu?o-Lizarán I, et al. Retinal α-synuclein
deposits in Parkinson's disease patients and animal models[ J]. Acta
Neuropathol, 2019, 137(3): 379-395.
53、Kaehler K, Seitter H, Sandbichler AM, et al. Assessment of the retina of plp-α-syn mice as a model for studying synuclein-dependent
diseases[ J]. Invest Ophthalmol Vis Sci, 2020, 61(6): 12.Kaehler K, Seitter H, Sandbichler AM, et al. Assessment of the retina of plp-α-syn mice as a model for studying synuclein-dependent
diseases[ J]. Invest Ophthalmol Vis Sci, 2020, 61(6): 12.
54、Tsai Y, Lu B, Ljubimov AV, et al. Ocular changes in TgF344-AD rat
model of Alzheimer's disease[ J]. Invest Ophthalmol Vis Sci, 2014,
55(1): 523-534.Tsai Y, Lu B, Ljubimov AV, et al. Ocular changes in TgF344-AD rat
model of Alzheimer's disease[ J]. Invest Ophthalmol Vis Sci, 2014,
55(1): 523-534.
55、Marrocco E, Indrieri A, Esposito F, et al. α-synuclein overexpression
in the retina leads to vision impairment and degeneration of
dopaminergic amacrine cells[ J]. Sci Rep, 2020, 10(1): 9619.Marrocco E, Indrieri A, Esposito F, et al. α-synuclein overexpression
in the retina leads to vision impairment and degeneration of
dopaminergic amacrine cells[ J]. Sci Rep, 2020, 10(1): 9619.
56、Prasad A, Bharathi V, Sivalingam V, et al. Molecular mechanisms of
TDP-43 misfolding and pathology in amyotrophic lateral sclerosis[ J].
Front Mol Neurosci, 2019, 12: 25.Prasad A, Bharathi V, Sivalingam V, et al. Molecular mechanisms of
TDP-43 misfolding and pathology in amyotrophic lateral sclerosis[ J].
Front Mol Neurosci, 2019, 12: 25.
57、Pinarbasi ES, Ca?atay T, Fung HYJ, et al. Active nuclear import and
passive nuclear export are the primary determinants of TDP-43
localization[ J]. Sci Rep, 2018, 8(1): 7083.Pinarbasi ES, Ca?atay T, Fung HYJ, et al. Active nuclear import and
passive nuclear export are the primary determinants of TDP-43
localization[ J]. Sci Rep, 2018, 8(1): 7083.
58、de Boer EMJ, Orie VK, Williams T, et al. TDP-43 proteinopathies:
a new wave of neurodegenerative diseases[ J]. J Neurol Neurosurg
Psychiatry, 2020, 92(1): 86-95.de Boer EMJ, Orie VK, Williams T, et al. TDP-43 proteinopathies:
a new wave of neurodegenerative diseases[ J]. J Neurol Neurosurg
Psychiatry, 2020, 92(1): 86-95.
59、Birsa N, Bentham MP, Fratta P. Cytoplasmic functions of TDP-43 and
FUS and their role in ALS[ J]. Semin Cell Dev Biol, 2020, 99: 193-201.Birsa N, Bentham MP, Fratta P. Cytoplasmic functions of TDP-43 and
FUS and their role in ALS[ J]. Semin Cell Dev Biol, 2020, 99: 193-201.
60、Wood A, Gurfinkel Y, Polain N, et al. Molecular mechanisms underlying
TDP-43 pathology in cellular and animal models of ALS and FTLD[ J].
Int J Mol Sci, 2021, 22(9): 4705.Wood A, Gurfinkel Y, Polain N, et al. Molecular mechanisms underlying
TDP-43 pathology in cellular and animal models of ALS and FTLD[ J].
Int J Mol Sci, 2021, 22(9): 4705.
61、Cook CN, Wu Y, Odeh HM, et al. C9orf72 poly(GR) aggregation
induces TDP-43 proteinopathy[ J]. Sci Transl Med, 2020, 12(559):
eabb3774.Cook CN, Wu Y, Odeh HM, et al. C9orf72 poly(GR) aggregation
induces TDP-43 proteinopathy[ J]. Sci Transl Med, 2020, 12(559):
eabb3774.
62、Nicholson AM, Zhou X, Perkerson RB, et al. Loss of Tmem106b is
unable to ameliorate frontotemporal dementia-like phenotypes in
an AAV mouse model of C9ORF72-repeat induced toxicity[ J]. Acta
Neuropathol Commun, 2018, 6(1): 42.Nicholson AM, Zhou X, Perkerson RB, et al. Loss of Tmem106b is
unable to ameliorate frontotemporal dementia-like phenotypes in
an AAV mouse model of C9ORF72-repeat induced toxicity[ J]. Acta
Neuropathol Commun, 2018, 6(1): 42.
63、Fawzi AA, Simonett JM, Purta P, et al. Clinicopathologic report
of ocular involvement in ALS patients with C9orf72 mutation[ J].
Amyotroph Lateral Scler Frontotemporal Degener, 2014, 15(7/8):
569-580.Fawzi AA, Simonett JM, Purta P, et al. Clinicopathologic report
of ocular involvement in ALS patients with C9orf72 mutation[ J].
Amyotroph Lateral Scler Frontotemporal Degener, 2014, 15(7/8):
569-580.
64、Atkinson R, Leung J, Bender J, et al. TDP-43 mislocalization drives
neurofilament changes in a novel model of TDP-43 proteinopathy[ J].
Dis Model Mech, 2021, 14(2): dmm047548.Atkinson R, Leung J, Bender J, et al. TDP-43 mislocalization drives
neurofilament changes in a novel model of TDP-43 proteinopathy[ J].
Dis Model Mech, 2021, 14(2): dmm047548.
65、Ward M E, Taubes A, Chen R, et al. Early retinal neurodegeneration
and impaired Ran-mediated nuclear import of TDP-43 in progranulin-deficient FTLD[ J]. J Exp Med, 2014, 211(10): 1937-1945.Ward M E, Taubes A, Chen R, et al. Early retinal neurodegeneration
and impaired Ran-mediated nuclear import of TDP-43 in progranulin-deficient FTLD[ J]. J Exp Med, 2014, 211(10): 1937-1945.
66、Li Y, R ay P, R ao EJ, et al. A drosophila model for TDP-43
proteinopathy[ J]. Proc Natl Acad Sci USA, 2010, 107(7): 3169-3174.Li Y, R ay P, R ao EJ, et al. A drosophila model for TDP-43
proteinopathy[ J]. Proc Natl Acad Sci USA, 2010, 107(7): 3169-3174.
67、Miguel L, Frébourg T, Campion D, et al. Both cytoplasmic and nuclear
accumulations of the protein are neurotoxic in Drosophila models of
TDP-43 proteinopathies[ J]. Neurobiol Dis, 2011, 41(2): 398-406.Miguel L, Frébourg T, Campion D, et al. Both cytoplasmic and nuclear
accumulations of the protein are neurotoxic in Drosophila models of
TDP-43 proteinopathies[ J]. Neurobiol Dis, 2011, 41(2): 398-406.
68、Ihara R , Matsukawa K , Nagata Y, et al. RNA binding mediates
neurotoxicity in the transgenic Drosophila model of TDP-43
proteinopathy[ J]. Hum Mol Genet, 2013, 22(22): 4474-4484.Ihara R , Matsukawa K , Nagata Y, et al. RNA binding mediates
neurotoxicity in the transgenic Drosophila model of TDP-43
proteinopathy[ J]. Hum Mol Genet, 2013, 22(22): 4474-4484.
69、Chou CC, Zhang Y, Umoh ME, et al. TDP-43 pathology disrupts
nuclear pore complexes and nucleocytoplasmic transport in ALS/
FTD[ J]. Nat Neurosci, 2018, 21(2): 228-239Chou CC, Zhang Y, Umoh ME, et al. TDP-43 pathology disrupts
nuclear pore complexes and nucleocytoplasmic transport in ALS/
FTD[ J]. Nat Neurosci, 2018, 21(2): 228-239
70、Fallini C, Khalil B, Smith CL, et al. Traffic jam at the nuclear pore: all
roads lead to nucleocytoplasmic transport defects in ALS/FTD[ J].
Neurobiol Dis, 2020, 140: 104835.Fallini C, Khalil B, Smith CL, et al. Traffic jam at the nuclear pore: all
roads lead to nucleocytoplasmic transport defects in ALS/FTD[ J].
Neurobiol Dis, 2020, 140: 104835.
71、Caviston JP, Holzbaur ELF. Huntingtin as an essential integrator of
intracellular vesicular trafficking[ J]. Trends Cell Biol, 2009, 19(4):
147-155.Caviston JP, Holzbaur ELF. Huntingtin as an essential integrator of
intracellular vesicular trafficking[ J]. Trends Cell Biol, 2009, 19(4):
147-155.
72、Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an
alternative approach to Huntington's disease[ J]. Nat Rev Neurosci,
2005, 6(12): 919-930.Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an
alternative approach to Huntington's disease[ J]. Nat Rev Neurosci,
2005, 6(12): 919-930.
73、Takahashi T, Katada S, Onodera O. Polyglutamine diseases: where does
toxicity come from? what is toxicity? where are we going?[ J]. J Mol
Cell Biol, 2010, 2(4): 180-191.Takahashi T, Katada S, Onodera O. Polyglutamine diseases: where does
toxicity come from? what is toxicity? where are we going?[ J]. J Mol
Cell Biol, 2010, 2(4): 180-191.
74、Batcha AH, Greferath U, Jobling AI, et al. Retinal dysfunction,
photoreceptor protein dysregulation and neuronal remodelling in the
R6/1 mouse model of Huntington's disease[ J]. Neurobiol Dis, 2012,
45(3): 887-896.Batcha AH, Greferath U, Jobling AI, et al. Retinal dysfunction,
photoreceptor protein dysregulation and neuronal remodelling in the
R6/1 mouse model of Huntington's disease[ J]. Neurobiol Dis, 2012,
45(3): 887-896.
75、Ragauskas S, Leinonen H, Puranen J, et al. Early retinal function deficit
without prominent morphological changes in the R6/2 mouse model
of Huntington's disease[ J]. PLoS One, 2014, 9(12): e113317.Ragauskas S, Leinonen H, Puranen J, et al. Early retinal function deficit
without prominent morphological changes in the R6/2 mouse model
of Huntington's disease[ J]. PLoS One, 2014, 9(12): e113317.
76、Helmlinger D, Yvert G, Picaud S, et al. Progressive retinal degeneration
and dysfunction in R6 Huntington's disease mice[ J]. Hum Mol Genet,
2002, 11(26): 3351-3359.Helmlinger D, Yvert G, Picaud S, et al. Progressive retinal degeneration
and dysfunction in R6 Huntington's disease mice[ J]. Hum Mol Genet,
2002, 11(26): 3351-3359.
77、Petrasch-Parwez E, Habbes HW, Weickert S, et al. Fine-structural
analysis and connexin expression in the retina of a transgenic model of
Huntington's disease[ J]. J Comp Neurol, 2004, 479(2): 181-197.Petrasch-Parwez E, Habbes HW, Weickert S, et al. Fine-structural
analysis and connexin expression in the retina of a transgenic model of
Huntington's disease[ J]. J Comp Neurol, 2004, 479(2): 181-197.
78、Karam A, Tebbe L, Weber C, et al. A novel function of Huntingtin
in the cilium and retinal ciliopathy in Huntington's disease mice[ J].
Neurobiol Dis, 2015, 80: 15-28.Karam A, Tebbe L, Weber C, et al. A novel function of Huntingtin
in the cilium and retinal ciliopathy in Huntington's disease mice[ J].
Neurobiol Dis, 2015, 80: 15-28.
79、Li M, Yasumura D, Ma AAK, et al. Intravitreal administration of HA-
1077, a ROCK inhibitor, improves retinal function in a mouse model of
Huntington disease[ J]. PLoS One, 2013, 8(2): e56026.Li M, Yasumura D, Ma AAK, et al. Intravitreal administration of HA-
1077, a ROCK inhibitor, improves retinal function in a mouse model of
Huntington disease[ J]. PLoS One, 2013, 8(2): e56026.
80、Cai W, Zhang K, Li P, et al. Dysfunction of the neurovascular unit in
ischemic stroke and neurodegenerative diseases: an aging effect[ J].
Ageing Res Rev, 2017, 34: 77-87.Cai W, Zhang K, Li P, et al. Dysfunction of the neurovascular unit in
ischemic stroke and neurodegenerative diseases: an aging effect[ J].
Ageing Res Rev, 2017, 34: 77-87.
81、Yu X , Ji C, Shao A . Neurovascular unit dysfunction and
neurodegenerative disorders[ J]. Front Neurosci, 2020, 14: 334.Yu X , Ji C, Shao A . Neurovascular unit dysfunction and
neurodegenerative disorders[ J]. Front Neurosci, 2020, 14: 334.
82、Sweeney MD, Kisler K, Montagne A, et al. The role of brain vasculature
in neurodegenerative disorders[ J]. Nat Neurosci, 2018, 21(10): 1318-
1331.Sweeney MD, Kisler K, Montagne A, et al. The role of brain vasculature
in neurodegenerative disorders[ J]. Nat Neurosci, 2018, 21(10): 1318-
1331.
83、Zlokov ic BV. The blood-brain barrier in health and chronic
neurodegenerative disorders[ J]. Neuron, 2008, 57(2): 178-201.Zlokov ic BV. The blood-brain barrier in health and chronic
neurodegenerative disorders[ J]. Neuron, 2008, 57(2): 178-201.
84、Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation
and neurovascular dysfunction in Alzheimer disease[ J]. Nat Rev
Neurosci, 2017, 18(7): 419-434.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation
and neurovascular dysfunction in Alzheimer disease[ J]. Nat Rev
Neurosci, 2017, 18(7): 419-434.
85、Saido TC, Iwata N. Metabolism of amyloid beta peptide and
pathogenesis of Alzheimer 's disease. Towards presymptomatic
diagnosis, prevention and therapy[ J]. Neurosci Res, 2006, 54(4): 235-
253.Saido TC, Iwata N. Metabolism of amyloid beta peptide and
pathogenesis of Alzheimer 's disease. Towards presymptomatic
diagnosis, prevention and therapy[ J]. Neurosci Res, 2006, 54(4): 235-
253.
86、Spires-Jones TL, Attems J, Thal DR. Interactions of pathological
proteins in neurodegenerative diseases[ J]. Acta Neuropathol, 2017,
134(2): 187-205Spires-Jones TL, Attems J, Thal DR. Interactions of pathological
proteins in neurodegenerative diseases[ J]. Acta Neuropathol, 2017,
134(2): 187-205
87、Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer�like neurodegeneration in mice[ J]. Nat Commun, 2013, 4: 2932.Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer�like neurodegeneration in mice[ J]. Nat Commun, 2013, 4: 2932.
88、Shi H, Koronyo Y, Rentsendorj A, et al. Identification of early pericyte
loss and vascular amyloidosis in Alzheimer's disease retina[ J]. Acta
Neuropathol, 2020, 139(5): 813-836.Shi H, Koronyo Y, Rentsendorj A, et al. Identification of early pericyte
loss and vascular amyloidosis in Alzheimer's disease retina[ J]. Acta
Neuropathol, 2020, 139(5): 813-836.
89、Shi H, Koronyo Y, Fuchs DT, et al. Retinal capillary degeneration
and blood-retinal barrier disruption in murine models of Alzheimer's
disease[ J]. Acta Neuropathol Commun, 2020, 8(1): 202.Shi H, Koronyo Y, Fuchs DT, et al. Retinal capillary degeneration
and blood-retinal barrier disruption in murine models of Alzheimer's
disease[ J]. Acta Neuropathol Commun, 2020, 8(1): 202.
90、Colonna M, Butovsky O. Microglia function in the central nervous
system during health and neurodegeneration[ J]. Annu Rev Immunol,
2017, 35: 441-468.Colonna M, Butovsky O. Microglia function in the central nervous
system during health and neurodegeneration[ J]. Annu Rev Immunol,
2017, 35: 441-468.
91、Cuenca N, Fernández-Sánchez L, Campello L, et al. Cellular
responses following retinal injuries and therapeutic approaches for
neurodegenerative diseases[ J]. Prog Retin Eye Res, 2014, 43: 17-75.Cuenca N, Fernández-Sánchez L, Campello L, et al. Cellular
responses following retinal injuries and therapeutic approaches for
neurodegenerative diseases[ J]. Prog Retin Eye Res, 2014, 43: 17-75.
92、Condello C, Yuan P, Schain A, et al. Microglia constitute a barrier that
prevents neurotoxic protofibrillar Aβ42 hotspots around plaques[ J].
Nat Commun, 2015, 6: 6176.Condello C, Yuan P, Schain A, et al. Microglia constitute a barrier that
prevents neurotoxic protofibrillar Aβ42 hotspots around plaques[ J].
Nat Commun, 2015, 6: 6176.
93、Yeh FL, Wang Y, Tom I, et al. TREM2 binds to apolipoproteins,
including APOE and CLU/APOJ, and thereby facilitates uptake of
amyloid-beta by microglia[ J]. Neuron, 2016, 91(2): 328-340.Yeh FL, Wang Y, Tom I, et al. TREM2 binds to apolipoproteins,
including APOE and CLU/APOJ, and thereby facilitates uptake of
amyloid-beta by microglia[ J]. Neuron, 2016, 91(2): 328-340.
94、Mazaheri F, Snaidero N, Kleinberger G, et al. TREM2 deficiency
impairs chemotaxis and microglial responses to neuronal injury[ J].
EMBO Rep, 2017, 18(7): 1186-1198Mazaheri F, Snaidero N, Kleinberger G, et al. TREM2 deficiency
impairs chemotaxis and microglial responses to neuronal injury[ J].
EMBO Rep, 2017, 18(7): 1186-1198
95、Atagi Y, Liu CC, Painter MM, et al. Apolipoprotein E is a ligand for
triggering receptor expressed on myeloid cells 2 (TREM2)[ J]. J Biol
Chem, 2015, 290(43): 26043-26050.Atagi Y, Liu CC, Painter MM, et al. Apolipoprotein E is a ligand for
triggering receptor expressed on myeloid cells 2 (TREM2)[ J]. J Biol
Chem, 2015, 290(43): 26043-26050.
96、Kamen LA, Levinsohn J, Swanson J A. Differential association of
phosphatidylinositol 3-kinase, SHIP-1, and PTEN with forming
phagosomes[ J]. Mol Biol Cell, 2007, 18(7): 2463-2472.Kamen LA, Levinsohn J, Swanson J A. Differential association of
phosphatidylinositol 3-kinase, SHIP-1, and PTEN with forming
phagosomes[ J]. Mol Biol Cell, 2007, 18(7): 2463-2472.
97、Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type
associated with restricting development of alzheimer's disease[ J]. Cell,
2017, 169(7): 1276-1290.e17.Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type
associated with restricting development of alzheimer's disease[ J]. Cell,
2017, 169(7): 1276-1290.e17.
98、Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer's
disease[ J]. Nat Immunol, 2015, 16(3): 229-236.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer's
disease[ J]. Nat Immunol, 2015, 16(3): 229-236.
99、Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in
Alzheimer's disease and contributes to pathology in APP/PS1 mice[ J].
Nature, 2013, 493(7434): 674-678.Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in
Alzheimer's disease and contributes to pathology in APP/PS1 mice[ J].
Nature, 2013, 493(7434): 674-678.
100、Ye L, Huang Y, Zhao L, et al. IL-1β and TNF-α induce neurotoxicity
through glutamate production: a potential role for neuronal
glutaminase[ J]. J Neurochem, 2013, 125(6): 897-908.Ye L, Huang Y, Zhao L, et al. IL-1β and TNF-α induce neurotoxicity
through glutamate production: a potential role for neuronal
glutaminase[ J]. J Neurochem, 2013, 125(6): 897-908.
101、Brown GC, Vilalta A. How microglia kill neurons[ J]. Brain Res, 2015,
1628: 288-297.Brown GC, Vilalta A. How microglia kill neurons[ J]. Brain Res, 2015,
1628: 288-297.
102、Kim JY, Kim N, Yenari MA. Mechanisms and potential therapeutic
applications of microglial activation after brain injury[ J]. CNS
Neurosci Ther, 2015, 21(4): 309-319.Kim JY, Kim N, Yenari MA. Mechanisms and potential therapeutic
applications of microglial activation after brain injury[ J]. CNS
Neurosci Ther, 2015, 21(4): 309-319.
103、Hansen DV, Hanson JE, Sheng M. Microglia in alzheimer's disease[ J]. J
Cell Biol, 2018, 217(2): 459-472.Hansen DV, Hanson JE, Sheng M. Microglia in alzheimer's disease[ J]. J
Cell Biol, 2018, 217(2): 459-472.
104、Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive
astrocytes are induced by activated microglia[ J]. Nature, 2017,
541(7638): 481-487.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive
astrocytes are induced by activated microglia[ J]. Nature, 2017,
541(7638): 481-487.
105、Grimaldi A, Pediconi N, Oieni F, et al. Neuroinflammatory processes,
A1 astrocyte activation and protein aggregation in the retina of
alzheimer's disease patients, possible biomarkers for early diagnosis[ J].
Front Neurosci, 2019, 13: 925.Grimaldi A, Pediconi N, Oieni F, et al. Neuroinflammatory processes,
A1 astrocyte activation and protein aggregation in the retina of
alzheimer's disease patients, possible biomarkers for early diagnosis[ J].
Front Neurosci, 2019, 13: 925.
106、Salobrar-García E, Rodrigues-Neves AC, Ramírez AI, et al. Microglial
activation in the retina of a triple-transgenic alzheimer's disease mouse
model (3xTg-AD) [ J]. Int J Mol Sci, 2020, 21(3): 816.Salobrar-García E, Rodrigues-Neves AC, Ramírez AI, et al. Microglial
activation in the retina of a triple-transgenic alzheimer's disease mouse
model (3xTg-AD) [ J]. Int J Mol Sci, 2020, 21(3): 816.
107、Grimaldi A, Brighi C, Peruzzi G, et al. Inflammation, neurodegeneration
and protein aggregation in the retina as ocular biomarkers for
Alzheimer's disease in the 3xTg-AD mouse model[ J]. Cell Death Dis,
2018, 9(6): 685.Grimaldi A, Brighi C, Peruzzi G, et al. Inflammation, neurodegeneration
and protein aggregation in the retina as ocular biomarkers for
Alzheimer's disease in the 3xTg-AD mouse model[ J]. Cell Death Dis,
2018, 9(6): 685.
108、Saidha S, Al-Louzi O, R atchford JN, et al. Optical coherence
tomography reflects brain atrophy in multiple sclerosis: a four-year
study[ J]. Ann Neurol, 2015, 78(5): 801-813.Saidha S, Al-Louzi O, R atchford JN, et al. Optical coherence
tomography reflects brain atrophy in multiple sclerosis: a four-year
study[ J]. Ann Neurol, 2015, 78(5): 801-813.
109、Sotirchos ES, Saidha S. OCT is an alternative to MRI for monitoring
MS - YES[ J]. Mult Scler, 2018, 24(6): 701-703.Sotirchos ES, Saidha S. OCT is an alternative to MRI for monitoring
MS - YES[ J]. Mult Scler, 2018, 24(6): 701-703.
110、Sabahi M, Joshaghanian A, Dolatshahi M, et al. Modification of glial cell
activation through dendritic cell vaccination: promises for treatment
of neurodegenerative diseases[ J]. J Mol Neurosci, 2021, 71(7): 1410-
1424.Sabahi M, Joshaghanian A, Dolatshahi M, et al. Modification of glial cell
activation through dendritic cell vaccination: promises for treatment
of neurodegenerative diseases[ J]. J Mol Neurosci, 2021, 71(7): 1410-
1424.
111、Liu X, Hou D, Lin F, et al. The role of neurovascular unit damage in the occurrence and development of Alzheimer's disease[ J]. Rev Neurosci,
2019, 30(5): 477-484.Liu X, Hou D, Lin F, et al. The role of neurovascular unit damage in the occurrence and development of Alzheimer's disease[ J]. Rev Neurosci,
2019, 30(5): 477-484.
112、Zipser BD, Johanson CE, Gonzalez L, et al. Microvascular injury and
blood-brain barrier leakage in Alzheimer's disease[ J]. Neurobiol Aging,
2007, 28(7): 977-986.Zipser BD, Johanson CE, Gonzalez L, et al. Microvascular injury and
blood-brain barrier leakage in Alzheimer's disease[ J]. Neurobiol Aging,
2007, 28(7): 977-986.
113、Baksi S, Singh N. α-Synuclein impairs ferritinophagy in the retinal
pigment epithelium: implications for retinal iron dyshomeostasis in
Parkinson's disease[ J]. Sci Rep, 2017, 7(1): 12843.Baksi S, Singh N. α-Synuclein impairs ferritinophagy in the retinal
pigment epithelium: implications for retinal iron dyshomeostasis in
Parkinson's disease[ J]. Sci Rep, 2017, 7(1): 12843.
114、Da Mesquita S, Papadopoulos Z, Dykstra T, et al. Meningeal lymphatics
affect microglia responses and anti-Aβ immunotherapy[ J]. Nature,
2021, 593(7858): 255-260.Da Mesquita S, Papadopoulos Z, Dykstra T, et al. Meningeal lymphatics
affect microglia responses and anti-Aβ immunotherapy[ J]. Nature,
2021, 593(7858): 255-260.
115、Ding XB, Wang XX, Xia DH, et al. Impaired meningeal lymphatic
drainage in patients with idiopathic Parkinson's disease[ J]. Nat Med,
2021, 27(3): 411-418.Ding XB, Wang XX, Xia DH, et al. Impaired meningeal lymphatic
drainage in patients with idiopathic Parkinson's disease[ J]. Nat Med,
2021, 27(3): 411-418.
116、Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major
neurodegenerative diseases[ J]. Int J Mol Sci, 2018, 19(10): 3082.Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major
neurodegenerative diseases[ J]. Int J Mol Sci, 2018, 19(10): 3082.
117、Fricker M, Tolkovsky AM, Borutaite V, et al.Neuronal Cell Death[ J].
Physiol Rev, 2018, 98(2):813-880.Fricker M, Tolkovsky AM, Borutaite V, et al.Neuronal Cell Death[ J].
Physiol Rev, 2018, 98(2):813-880.
118、Hickman S, Izzy S, Sen P, et al. Microglia in neurodegeneration[ J]. Nat
Neurosci, 2018, 21(10): 1359-1369.Hickman S, Izzy S, Sen P, et al. Microglia in neurodegeneration[ J]. Nat
Neurosci, 2018, 21(10): 1359-1369.
119、Sadun AA, Bassi CJ. Optic nerve damage in alzheimer's disease[ J].
Ophthalmology, 1990, 97(1): 9-17.Sadun AA, Bassi CJ. Optic nerve damage in alzheimer's disease[ J].
Ophthalmology, 1990, 97(1): 9-17.
120、Syed AB, Armstrong RA, Smith CUM. A quantitative analysis of optic
nerve axons in elderly control subjects and patients with Alzheimer's
disease[ J]. Folia Neuropathol, 2005, 43(1): 1-6.Syed AB, Armstrong RA, Smith CUM. A quantitative analysis of optic
nerve axons in elderly control subjects and patients with Alzheimer's
disease[ J]. Folia Neuropathol, 2005, 43(1): 1-6.
121、Masri RA, Grünert U, Martin PR . Analysis of parvocellular and
magnocellular visual pathways in human retina[ J]. J Neurosci, 2020,
40(42): 8132-8148.Masri RA, Grünert U, Martin PR . Analysis of parvocellular and
magnocellular visual pathways in human retina[ J]. J Neurosci, 2020,
40(42): 8132-8148.
122、Cerquera-Jaramillo MA, Nava-Mesa MO, González-Reyes R E, et al.
Visual features in alzheimer's disease: from basic mechanisms to clinical
overview[ J]. Neural Plast, 2018, 2018: 2941783.Cerquera-Jaramillo MA, Nava-Mesa MO, González-Reyes R E, et al.
Visual features in alzheimer's disease: from basic mechanisms to clinical
overview[ J]. Neural Plast, 2018, 2018: 2941783.
123、Wright CE, Drasdo N, Harding GF. Pathology of the optic nerve and
visual association areas. Information given by the flash and pattern
visual evoked potential, and the temporal and spatial contrast sensitivity
function[ J]. Brain, 1987, 110( Pt 1): 107-120.Wright CE, Drasdo N, Harding GF. Pathology of the optic nerve and
visual association areas. Information given by the flash and pattern
visual evoked potential, and the temporal and spatial contrast sensitivity
function[ J]. Brain, 1987, 110( Pt 1): 107-120.
124、Pache, Smeets CH, Gasio PF, et al. Colour vision deficiencies in
Alzheimer's disease[ J]. Age Ageing, 2003, 32(4): 422-426.Pache, Smeets CH, Gasio PF, et al. Colour vision deficiencies in
Alzheimer's disease[ J]. Age Ageing, 2003, 32(4): 422-426.
125、葛坚, 王宁利.眼科学[M]. 第三版, 人民卫生出版社, 2016: 80.
Ge J, Wang NL. Ophthalmology[M], Third edition, People's Medical
Publishing House, 2016:80葛坚, 王宁利.眼科学[M]. 第三版, 人民卫生出版社, 2016: 80.
Ge J, Wang NL. Ophthalmology[M], Third edition, People's Medical
Publishing House, 2016:80
126、Vujosevic S, Parra M M, Hartnett M E, et al. Optical coherence
tomography as retinal imaging biomarker of neuroinflammation/
neurodegeneration in systemic disorders in adults and children[ J]. Eye
(Lond), 2023, 37(2): 203-219.Vujosevic S, Parra M M, Hartnett M E, et al. Optical coherence
tomography as retinal imaging biomarker of neuroinflammation/
neurodegeneration in systemic disorders in adults and children[ J]. Eye
(Lond), 2023, 37(2): 203-219.
127、Ochi H, Fujihara K. Demyelinating diseases in Asia[ J]. Curr Opin
Neurol, 2016, 29(3): 222-228.Ochi H, Fujihara K. Demyelinating diseases in Asia[ J]. Curr Opin
Neurol, 2016, 29(3): 222-228.
128、Forsthuber TG, Cimbora DM, Ratchford JN, et al. B cell-based
therapies in CNS autoimmunity: differentiating CD19 and CD20
as therapeutic targets[ J]. Ther Adv Neurol Disord, 2018, 11:
1756286418761697.Forsthuber TG, Cimbora DM, Ratchford JN, et al. B cell-based
therapies in CNS autoimmunity: differentiating CD19 and CD20
as therapeutic targets[ J]. Ther Adv Neurol Disord, 2018, 11:
1756286418761697.
129、Dargahi N, K atsara M, Tselios T, et al. Multiple sclerosis:
immunopathology and treatment update[ J]. Brain Sci, 2017, 7(7): 78.Dargahi N, K atsara M, Tselios T, et al. Multiple sclerosis:
immunopathology and treatment update[ J]. Brain Sci, 2017, 7(7): 78.
130、Bramow S, Frischer JM, Lassmann H, et al. Demyelination versus
remyelination in progressive multiple sclerosis[ J]. Brain, 2010,
133(10): 2983-2998.Bramow S, Frischer JM, Lassmann H, et al. Demyelination versus
remyelination in progressive multiple sclerosis[ J]. Brain, 2010,
133(10): 2983-2998.
131、Blank T, Prinz M. Type I interferon pathway in CNS homeostasis and
neurological disorders[ J]. Glia, 2017, 65(9): 1397-1406.Blank T, Prinz M. Type I interferon pathway in CNS homeostasis and
neurological disorders[ J]. Glia, 2017, 65(9): 1397-1406.
132、中国免疫学会神经免疫分会. 中国视神经脊髓炎谱系疾病诊
断与治疗指南(2021版)[ J]. 中国神经免疫学和神经病学杂志,
2021, 28(6): 423-436.
Neuroimmunology Branch of Chinese Society of Immunology.
Chinese guidelines for diagnosis and treatment of neuromyelitis
optica spectrum diseases(2021 edition)[ J]. Chinese Journal of
Neuroimmunology and Neurology, 2021, 28(6): 423-436.中国免疫学会神经免疫分会. 中国视神经脊髓炎谱系疾病诊
断与治疗指南(2021版)[ J]. 中国神经免疫学和神经病学杂志,
2021, 28(6): 423-436.
Neuroimmunology Branch of Chinese Society of Immunology.
Chinese guidelines for diagnosis and treatment of neuromyelitis
optica spectrum diseases(2021 edition)[ J]. Chinese Journal of
Neuroimmunology and Neurology, 2021, 28(6): 423-436.
133、Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody
marker of neuromyelitis optica: distinction from multiple sclerosis[ J].
Lancet, 2004, 364(9451): 2106-2112.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody
marker of neuromyelitis optica: distinction from multiple sclerosis[ J].
Lancet, 2004, 364(9451): 2106-2112.
134、Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of
neuromyelitis optica[ J]. Lancet Neurol, 2007, 6(9): 805-815.Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of
neuromyelitis optica[ J]. Lancet Neurol, 2007, 6(9): 805-815.
135、Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte
glycoprotein antibody-associated disease[ J]. Lancet Neurol, 2021,
20(9): 762-772.Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte
glycoprotein antibody-associated disease[ J]. Lancet Neurol, 2021,
20(9): 762-772.