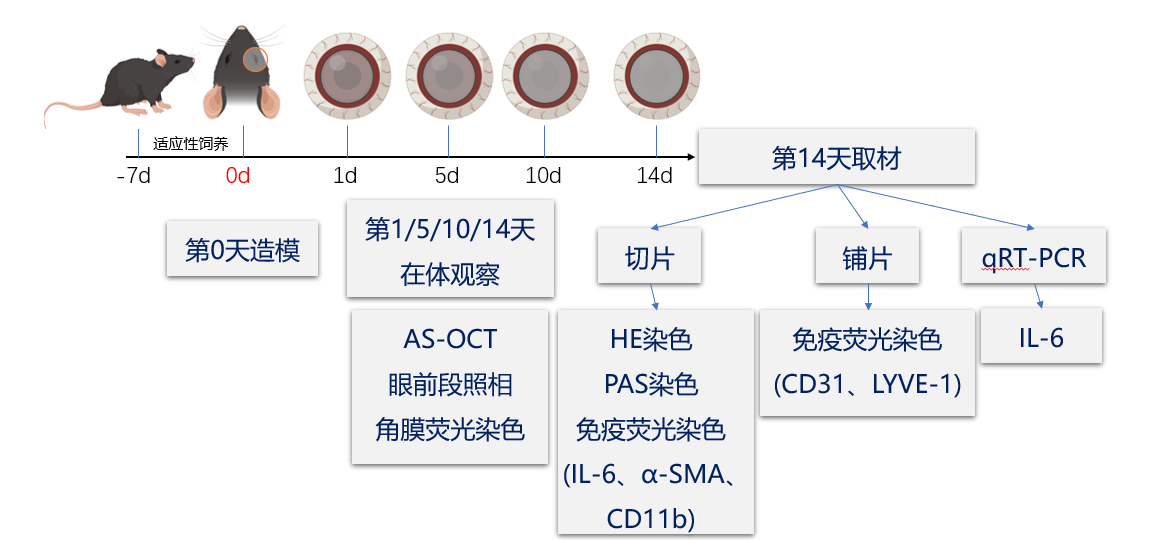
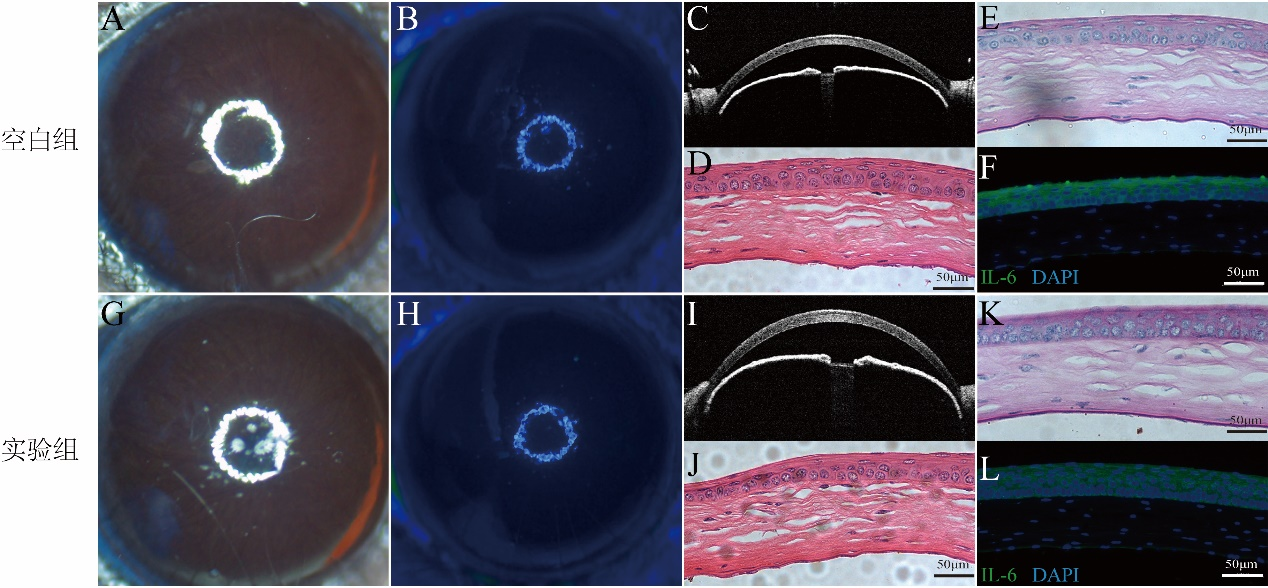
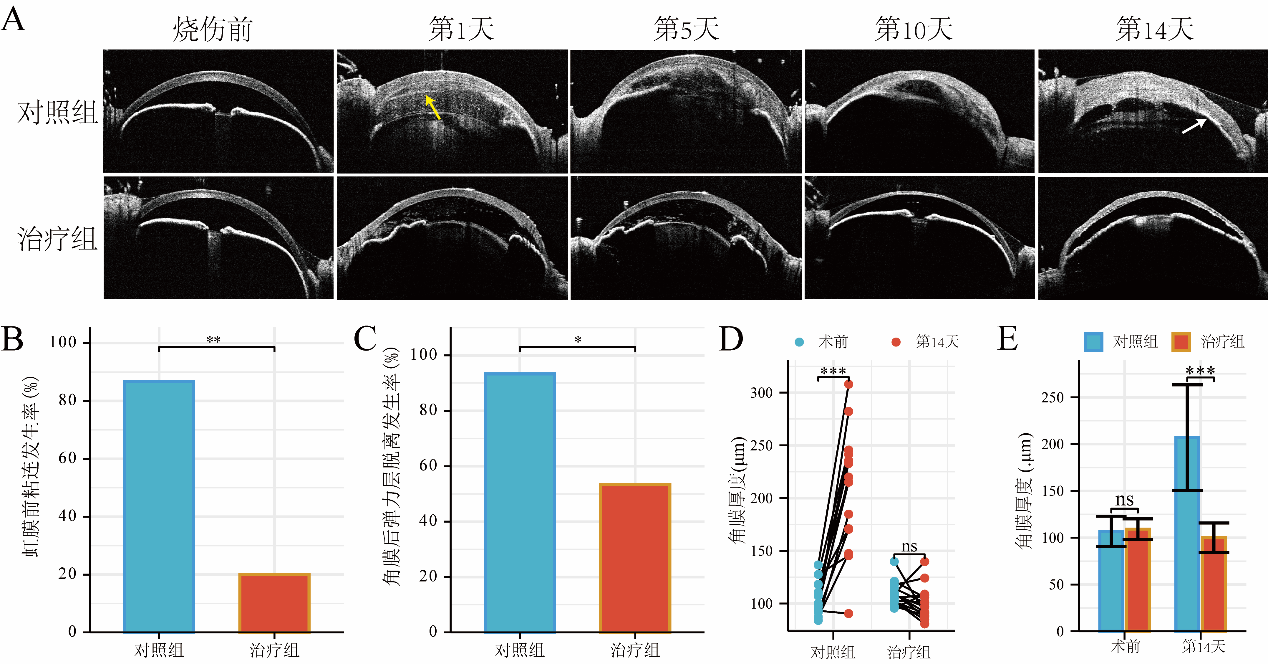
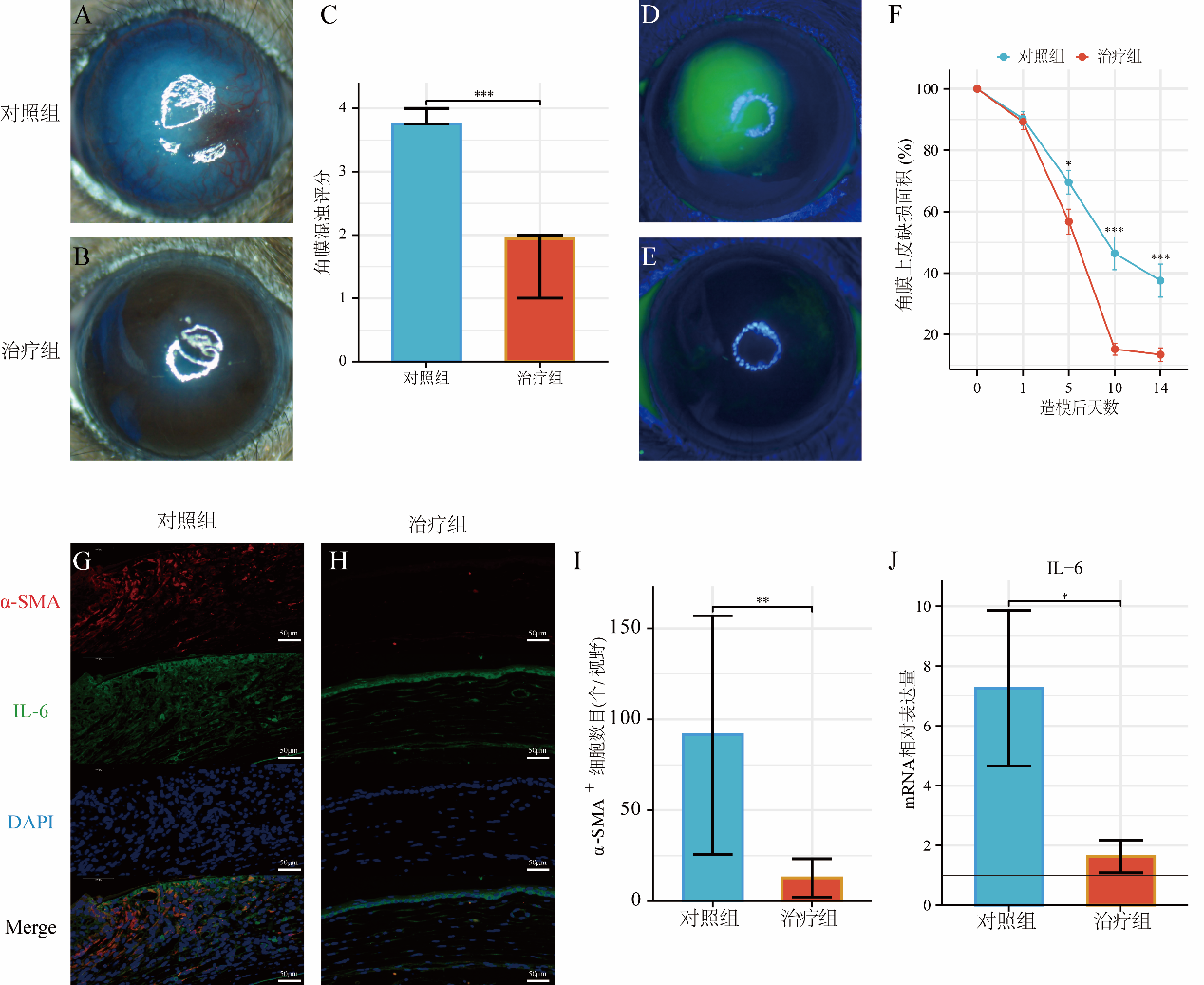
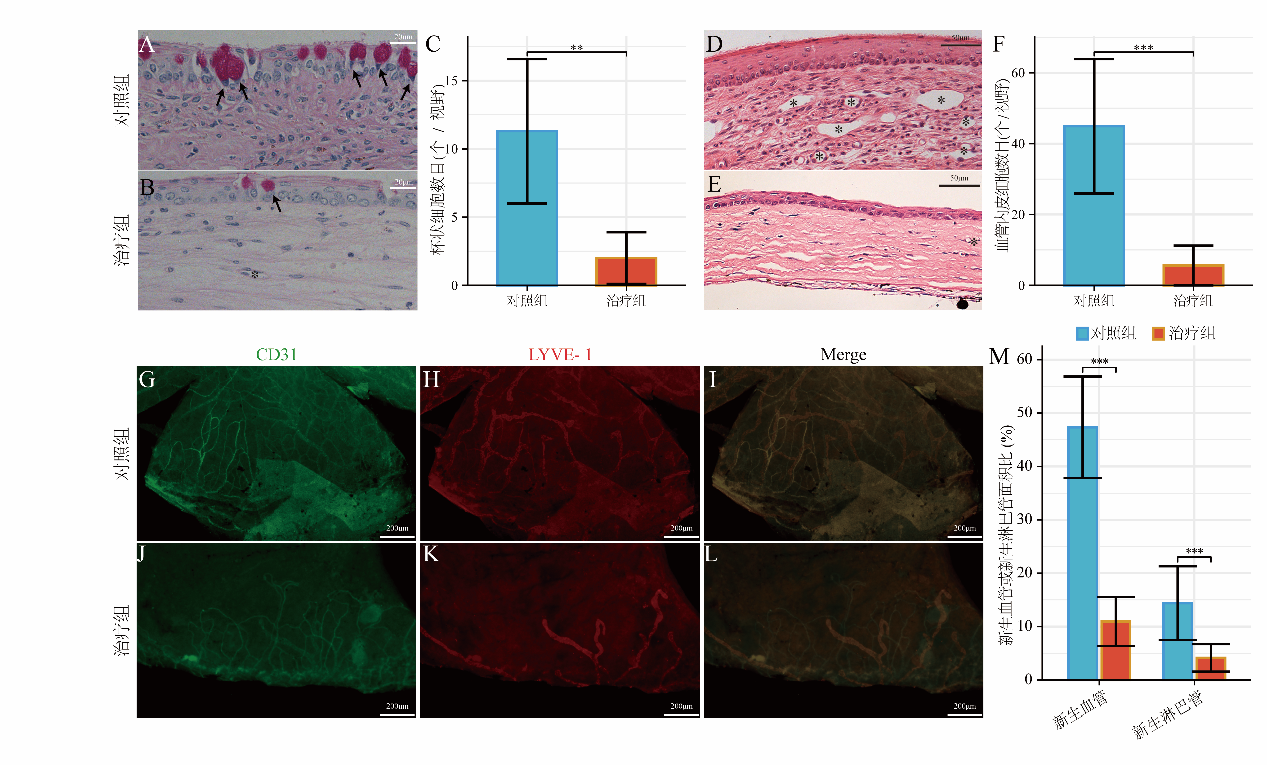
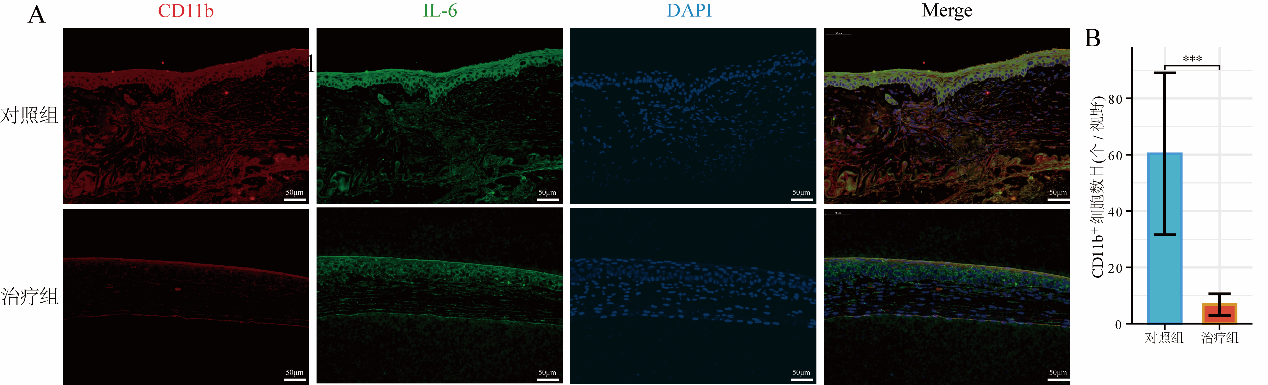
1、Wagoner MD. Chemical injuries of the eye: current concepts in
pathophysiology and therapy[ J]. Surv Ophthalmol, 1997, 41(4): 275-
313. DOI: 10.1016/s0039-6257(96)00007-0.Wagoner MD. Chemical injuries of the eye: current concepts in
pathophysiology and therapy[ J]. Surv Ophthalmol, 1997, 41(4): 275-
313. DOI: 10.1016/s0039-6257(96)00007-0.
2、Kate A, Sharma S, Yathish S, et al. Demographic profile and clinical
characteristics of patients presenting with acute ocular burns[ J].
Indian J Ophthalmol, 2023, 71(7): 2694-2703. DOI: 10.4103/IJO.
IJO_3330_22.Kate A, Sharma S, Yathish S, et al. Demographic profile and clinical
characteristics of patients presenting with acute ocular burns[ J].
Indian J Ophthalmol, 2023, 71(7): 2694-2703. DOI: 10.4103/IJO.
IJO_3330_22.
3、Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of
chemical and thermal eye burns[ J]. Acta Ophthalmol Scand, 2002,
80(1): 4-10. DOI: 10.1034/j.1600-0420.2002.800102.x.Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of
chemical and thermal eye burns[ J]. Acta Ophthalmol Scand, 2002,
80(1): 4-10. DOI: 10.1034/j.1600-0420.2002.800102.x.
4、Tran TM, Duong H, Bonnet C, et al. Corneal blindness in Asia:
a systematic review and meta-analysis to identify challenges and
opportunities[ J]. Cornea, 2020, 39(9): 1196-1205. DOI: 10.1097/
ICO.0000000000002374.Tran TM, Duong H, Bonnet C, et al. Corneal blindness in Asia:
a systematic review and meta-analysis to identify challenges and
opportunities[ J]. Cornea, 2020, 39(9): 1196-1205. DOI: 10.1097/
ICO.0000000000002374.
5、Williams%20KA%2C%20Esterman%20AJ%2C%20Bartlett%20C%2C%20et%20al.%20How%20effective%20is%20penetrating%20%0Acorneal%20transplantation%3F%20Factors%20influencing%20long-term%20outcome%20in%20%0Amultivariate%20analysis%5B%20J%5D.%20Transplantation%2C%202006%2C%2081(6)%3A%20896-901.%20DOI%3A%20%0A10.1097%2F01.tp.0000185197.37824.35.Williams%20KA%2C%20Esterman%20AJ%2C%20Bartlett%20C%2C%20et%20al.%20How%20effective%20is%20penetrating%20%0Acorneal%20transplantation%3F%20Factors%20influencing%20long-term%20outcome%20in%20%0Amultivariate%20analysis%5B%20J%5D.%20Transplantation%2C%202006%2C%2081(6)%3A%20896-901.%20DOI%3A%20%0A10.1097%2F01.tp.0000185197.37824.35.
6、Mohan RR, Kempuraj D, D'Souza S, et al. Corneal stromal repair and
regeneration[ J]. Prog Retin Eye Res, 2022, 91: 101090. DOI: 10.1016/
j.preteyeres.2022.101090.Mohan RR, Kempuraj D, D'Souza S, et al. Corneal stromal repair and
regeneration[ J]. Prog Retin Eye Res, 2022, 91: 101090. DOI: 10.1016/
j.preteyeres.2022.101090.
7、Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical
burns[ J]. Surv Ophthalmol, 2018, 63(2): 214-235. DOI: 10.1016/
j.survophthal.2017.09.005.Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical
burns[ J]. Surv Ophthalmol, 2018, 63(2): 214-235. DOI: 10.1016/
j.survophthal.2017.09.005.
8、Thathapudi NC, Groleau M, Degué DS, et al. Novel micellar CB2
receptor agonist with anti-inflammatory action for treating corneal
alkali burns in a mouse model[ J]. Front Pharmacol, 2023, 14: 1270699.
DOI: 10.3389/fphar.2023.1270699.Thathapudi NC, Groleau M, Degué DS, et al. Novel micellar CB2
receptor agonist with anti-inflammatory action for treating corneal
alkali burns in a mouse model[ J]. Front Pharmacol, 2023, 14: 1270699.
DOI: 10.3389/fphar.2023.1270699.
9、Donshik PC, Berman MB, Dohlman CH, et al. Effect of topical
corticosteroids on ulceration in alkali-burned corneas[ J]. Arch
Ophthalmol, 1978, 96(11): 2117-2120. DOI: 10.1001/archopht.1978.
03910060497024.Donshik PC, Berman MB, Dohlman CH, et al. Effect of topical
corticosteroids on ulceration in alkali-burned corneas[ J]. Arch
Ophthalmol, 1978, 96(11): 2117-2120. DOI: 10.1001/archopht.1978.
03910060497024.
10、Urru M, Ranieri G, Mencucci R , et al. Comparison of the anti�inflammatory and cytotoxic potential of different corticosteroid eye
drop preparations[ J]. Ocul Immunol Inflamm, 2020, 28(5): 839-845.
DOI: 10.1080/09273948.2019.1634214.Urru M, Ranieri G, Mencucci R , et al. Comparison of the anti�inflammatory and cytotoxic potential of different corticosteroid eye
drop preparations[ J]. Ocul Immunol Inflamm, 2020, 28(5): 839-845.
DOI: 10.1080/09273948.2019.1634214.
11、Hoffman RS, Braga-Mele R, Donaldson K, et al. Cataract surgery and
nonsteroidal antiinflammatory drugs[ J]. J Cataract Refract Surg, 2016,
42(9): 1368-1379. DOI: 10.1016/j.jcrs.2016.06.006.Hoffman RS, Braga-Mele R, Donaldson K, et al. Cataract surgery and
nonsteroidal antiinflammatory drugs[ J]. J Cataract Refract Surg, 2016,
42(9): 1368-1379. DOI: 10.1016/j.jcrs.2016.06.006.
12、Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine
and chemokine analysis and clinical correlations in evaporative-type
dry eye disease [ J]. Mol Vis. 2010;16:862-73. https://www.ncbi.nlm.
nih.gov/pubmed/20508732.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine
and chemokine analysis and clinical correlations in evaporative-type
dry eye disease [ J]. Mol Vis. 2010;16:862-73. https://www.ncbi.nlm.
nih.gov/pubmed/20508732.
13、di Girolamo N, Kumar RK , Coroneo MT, et al. UVB-mediated
induction of interleukin-6 and-8 in pterygia and cultured human
pterygium epithelial cells[ J]. Invest Ophthalmol Vis Sci, 2002, 43(11):
3430-3437.di Girolamo N, Kumar RK , Coroneo MT, et al. UVB-mediated
induction of interleukin-6 and-8 in pterygia and cultured human
pterygium epithelial cells[ J]. Invest Ophthalmol Vis Sci, 2002, 43(11):
3430-3437.
14、Lewis AC. Interleukin-6 in the pathogenesis of posterior capsule
opacification and the potential role for interleukin-6 inhibition in the
future of cataract surgery[ J]. Med Hypotheses, 2013, 80(4): 466-474.
DOI: 10.1016/j.mehy.2012.12.042.Lewis AC. Interleukin-6 in the pathogenesis of posterior capsule
opacification and the potential role for interleukin-6 inhibition in the
future of cataract surgery[ J]. Med Hypotheses, 2013, 80(4): 466-474.
DOI: 10.1016/j.mehy.2012.12.042.
15、G h a s e m i H . R o l e s o f I L - 6 i n o c u l a r i n f l a m m a t i o n : a
review[ J]. Ocul Immunol Inflamm, 2018, 26(1): 37-50. DOI:
10.1080/09273948.2016.1277247.G h a s e m i H . R o l e s o f I L - 6 i n o c u l a r i n f l a m m a t i o n : a
review[ J]. Ocul Immunol Inflamm, 2018, 26(1): 37-50. DOI:
10.1080/09273948.2016.1277247.
16、Chucair-Elliott AJ, Jinkins J, Carr MM, et al. IL -6 contributes
to corneal nerve degeneration after herpes simplex virus type I
infection[ J]. Am J Pathol, 2016, 186(10): 2665-2678. DOI: 10.1016/
j.ajpath.2016.06.007.Chucair-Elliott AJ, Jinkins J, Carr MM, et al. IL -6 contributes
to corneal nerve degeneration after herpes simplex virus type I
infection[ J]. Am J Pathol, 2016, 186(10): 2665-2678. DOI: 10.1016/
j.ajpath.2016.06.007.
17、Sakimoto T, Sugaya S, Ishimori A, et al. Anti-inflammatory effect of
IL-6 receptor blockade in corneal alkali burn[ J]. Exp Eye Res, 2012,
97(1): 98-104. DOI: 10.1016/j.exer.2012.02.015.Sakimoto T, Sugaya S, Ishimori A, et al. Anti-inflammatory effect of
IL-6 receptor blockade in corneal alkali burn[ J]. Exp Eye Res, 2012,
97(1): 98-104. DOI: 10.1016/j.exer.2012.02.015.
18、Arranz-Valsero I, Soriano-Romaní L, García-Posadas L, et al. IL-6 as a
corneal wound healing mediator in an in vitro scratch assay[ J]. Exp Eye
Res, 2014, 125: 183-192. DOI: 10.1016/j.exer.2014.06.012.Arranz-Valsero I, Soriano-Romaní L, García-Posadas L, et al. IL-6 as a
corneal wound healing mediator in an in vitro scratch assay[ J]. Exp Eye
Res, 2014, 125: 183-192. DOI: 10.1016/j.exer.2014.06.012.
19、Vílchez-Oya F, Sánchez-Schmidt JM, Agustí A, et al. The use of
tocilizumab in the treatment of refractory eosinophilic fasciitis: a case�based review[ J]. Clin Rheumatol, 2020, 39(5): 1693-1698. DOI:
10.1007/s10067-020-04952-5.Vílchez-Oya F, Sánchez-Schmidt JM, Agustí A, et al. The use of
tocilizumab in the treatment of refractory eosinophilic fasciitis: a case�based review[ J]. Clin Rheumatol, 2020, 39(5): 1693-1698. DOI:
10.1007/s10067-020-04952-5.
20、Avci%20AB%2C%20Feist%20E%2C%20Burmester%20GR.%20Targeting%20IL-6%20or%20IL-6%20receptor%20in%20%0Arheumatoid%20arthritis%3A%20what%20have%20we%20learned%3F%5B%20J%5D.%20BioDrugs%2C%202024%2C%20%0A38(1)%3A%2061-71.%20DOI%3A%2010.1007%2Fs40259-023-00634-1.Avci%20AB%2C%20Feist%20E%2C%20Burmester%20GR.%20Targeting%20IL-6%20or%20IL-6%20receptor%20in%20%0Arheumatoid%20arthritis%3A%20what%20have%20we%20learned%3F%5B%20J%5D.%20BioDrugs%2C%202024%2C%20%0A38(1)%3A%2061-71.%20DOI%3A%2010.1007%2Fs40259-023-00634-1.
21、Geng C, Zhao W, Wang Z, et al. Acute necrotizing encephalopathy
associated with COVID-19: case series and systematic review[ J].
J Neurol, 2023, 270(11): 5171-5181. DOI: 10.1007/s00415-023-
11915-8.Geng C, Zhao W, Wang Z, et al. Acute necrotizing encephalopathy
associated with COVID-19: case series and systematic review[ J].
J Neurol, 2023, 270(11): 5171-5181. DOI: 10.1007/s00415-023-
11915-8.
22、Mendoza FA, Allawh T, Jimenez SA. Pharmacological treatment of
systemic sclerosis-associated interstitial lung disease: an updated review
and current approach to patient care[ J]. Clin Exp Rheumatol, 2023,
41(8): 1704-1712. DOI: 10.55563/clinexprheumatol/am4nmv.Mendoza FA, Allawh T, Jimenez SA. Pharmacological treatment of
systemic sclerosis-associated interstitial lung disease: an updated review
and current approach to patient care[ J]. Clin Exp Rheumatol, 2023,
41(8): 1704-1712. DOI: 10.55563/clinexprheumatol/am4nmv.
23、ElIskandarani S, Khasho M, MerashliM. Tocilizumab in the treatment
of eosinophilic fasciitis: acase study and review of literature[ J].
MediterrJRheumatol, 2023, 34(1): 78-85. DOI: 10.31138/mjr.34.1.78.ElIskandarani S, Khasho M, MerashliM. Tocilizumab in the treatment
of eosinophilic fasciitis: acase study and review of literature[ J].
MediterrJRheumatol, 2023, 34(1): 78-85. DOI: 10.31138/mjr.34.1.78.
24、Jeong MK, Kim BH. Grading criteria of histopathological evaluation in
BCOP assay by various staining methods[ J]. Toxicol Res,2021, 38(1):
9-17. DOI: 10.1007/s43188-021-00099-w.Jeong MK, Kim BH. Grading criteria of histopathological evaluation in
BCOP assay by various staining methods[ J]. Toxicol Res,2021, 38(1):
9-17. DOI: 10.1007/s43188-021-00099-w.
25、Ong HS, Riau AK, Yam GH, et al. Mesenchymal stem cell exosomes
as immunomodulatory therapy for corneal scarring[ J]. Int J Mol Sci,
2023, 24(8): 7456. DOI: 10.3390/ijms24087456.Ong HS, Riau AK, Yam GH, et al. Mesenchymal stem cell exosomes
as immunomodulatory therapy for corneal scarring[ J]. Int J Mol Sci,
2023, 24(8): 7456. DOI: 10.3390/ijms24087456.
26、Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity,
and disease[ J]. Cold Spring Harb Perspect Biol, 2014, 6(10): a016295.
DOI: 10.1101/cshperspect.a016295.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity,
and disease[ J]. Cold Spring Harb Perspect Biol, 2014, 6(10): a016295.
DOI: 10.1101/cshperspect.a016295.
27、Ebihara N, Matsuda A, Nakamura S, et al. Role of the IL-6 classic- and
trans-signaling pathways in corneal sterile inflammation and wound
healing[ J]. Invest Ophthalmol Vis Sci, 2011, 52(12): 8549-8557. DOI:
10.1167/iovs.11-7956.Ebihara N, Matsuda A, Nakamura S, et al. Role of the IL-6 classic- and
trans-signaling pathways in corneal sterile inflammation and wound
healing[ J]. Invest Ophthalmol Vis Sci, 2011, 52(12): 8549-8557. DOI:
10.1167/iovs.11-7956.
28、Sugaya S, Sakimoto T, Shoji J, et al. Regulation of soluble interleukin-6
(IL-6) receptor release from corneal epithelial cells and its role in the
ocular surface[ J]. Jpn J Ophthalmol, 2011, 55(3): 277-282. DOI:
10.1007/s10384-011-0002-x.Sugaya S, Sakimoto T, Shoji J, et al. Regulation of soluble interleukin-6
(IL-6) receptor release from corneal epithelial cells and its role in the
ocular surface[ J]. Jpn J Ophthalmol, 2011, 55(3): 277-282. DOI:
10.1007/s10384-011-0002-x.
29、Yu B, Yang L, Song S, et al. LRG1 facilitates corneal fibrotic response
by inducing neutrophil chemotaxis via Stat3 signaling in alkali�burned mouse corneas[ J]. Am J Physiol Cell Physiol, 2021, 321(3):
C415-C428. DOI: 10.1152/ajpcell.00517.2020.Yu B, Yang L, Song S, et al. LRG1 facilitates corneal fibrotic response
by inducing neutrophil chemotaxis via Stat3 signaling in alkali�burned mouse corneas[ J]. Am J Physiol Cell Physiol, 2021, 321(3):
C415-C428. DOI: 10.1152/ajpcell.00517.2020.
30、McFarland-Mancini MM, Funk HM, Paluch AM, et al. Differences
in wound healing in mice with deficiency of IL -6 versus IL -6
receptor[ J]. J Immunol, 2010, 184(12): 7219-7228. DOI: 10.4049/
jimmunol.0901929.McFarland-Mancini MM, Funk HM, Paluch AM, et al. Differences
in wound healing in mice with deficiency of IL -6 versus IL -6
receptor[ J]. J Immunol, 2010, 184(12): 7219-7228. DOI: 10.4049/
jimmunol.0901929.
31、Geremicca W, Fonte C, Vecchio S. Blood components for topical use in
tissue regeneration: evaluation of corneal lesions treated with platelet
lysate and considerations on repair mechanisms[ J]. Blood Transfus,
2010, 8(2): 107-112. DOI: 10.2450/2009.0091-09.Geremicca W, Fonte C, Vecchio S. Blood components for topical use in
tissue regeneration: evaluation of corneal lesions treated with platelet
lysate and considerations on repair mechanisms[ J]. Blood Transfus,
2010, 8(2): 107-112. DOI: 10.2450/2009.0091-09.
32、Park SB, Jung SH, Jin H, et al. Bioluminescence imaging of matrix
metalloproteinases-2 and-9 activities in ethanol-injured cornea
of mice[ J]. In Vivo, 2021, 35(3): 1521-1528. DOI: 10.21873/
invivo.12405.Park SB, Jung SH, Jin H, et al. Bioluminescence imaging of matrix
metalloproteinases-2 and-9 activities in ethanol-injured cornea
of mice[ J]. In Vivo, 2021, 35(3): 1521-1528. DOI: 10.21873/
invivo.12405.
33、Zhou C, Singh A, Qian G, et al. Microporous drug delivery system for
sustained anti-VEGF delivery to the eye[ J]. Transl Vis Sci Technol,
2020, 9(8): 5. DOI: 10.1167/tvst.9.8.5.Zhou C, Singh A, Qian G, et al. Microporous drug delivery system for
sustained anti-VEGF delivery to the eye[ J]. Transl Vis Sci Technol,
2020, 9(8): 5. DOI: 10.1167/tvst.9.8.5.