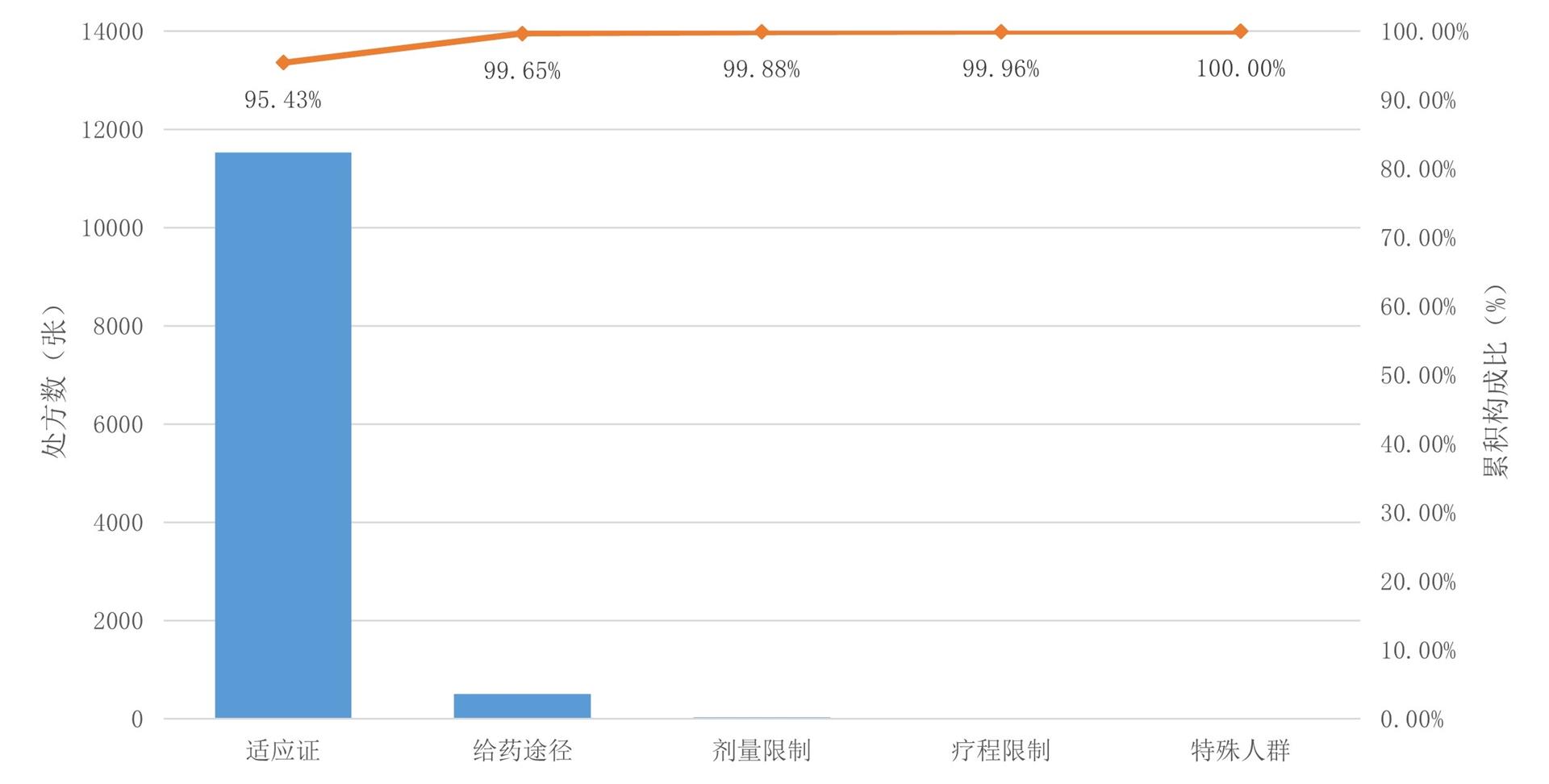
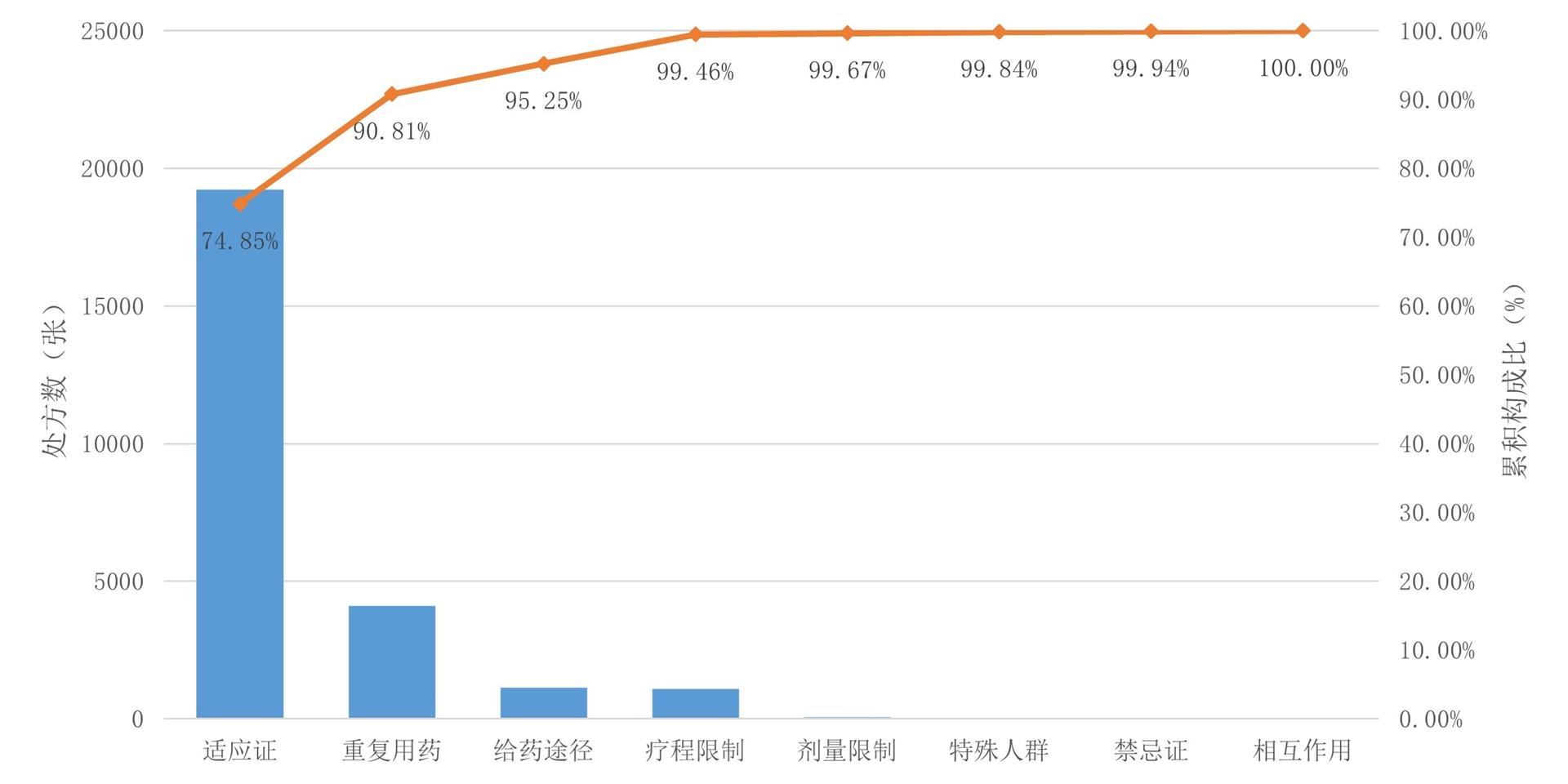
1、Kit Delgado M, Shofer FS, Patel MS, et al. Association between electronic medical record implementation of default opioid prescription quantities and prescribing behavior in two emergency departments[J]. J Gen Intern Med, 2018, 33(4): 409-411. DOI: 10.1007/s11606-017-4286-5. Kit Delgado M, Shofer FS, Patel MS, et al. Association between electronic medical record implementation of default opioid prescription quantities and prescribing behavior in two emergency departments[J]. J Gen Intern Med, 2018, 33(4): 409-411. DOI: 10.1007/s11606-017-4286-5.
2、处方管理办法(卫生部令第53号)[EB/OL]. (2007-03-13). https://www.gov.cn/flfg/2007-03/13/content_549406.htm. Regulations on the Administration of Prescriptions (Order No. 53 of the Ministry of Health) [EB/OL]. (2007-03-13). https://www.gov.cn/flfg/2007-03/13/content_549406.htm.Regulations on the Administration of Prescriptions (Order No. 53 of the Ministry of Health) [EB/OL]. (2007-03-13). https://www.gov.cn/flfg/2007-03/13/content_549406.htm.
3、关于印发医疗机构处方审核规范的通知[EB/OL]. (2018-06-29). https://www.gov.cn/zhengce/zhengceku/2018-12/31/content_5435182.htm.Notice on Issuing the Standards for Prescription Review in Medical Institutions. [EB/OL]. (2018-06-29). https://www.gov.cn/zhengce/zhengceku/2018-12/31/content_5435182.htm.Notice on Issuing the Standards for Prescription Review in Medical Institutions. [EB/OL]. (2018-06-29). https://www.gov.cn/zhengce/zhengceku/2018-12/31/content_5435182.htm.
4、卞婧, 魏丽艳, 邵晓楠, 等. 国内医院处方审核与点评开展情况及分析[J]. 中国医院, 2020, 24(2): 8-11. DOI: 10.19660/j.issn.1671-0592.2020.02.03.Bian J, Wei LY, Shao XN, et al. Investigation and analysis of prescription review and evaluation in Chinese hospitals[J]. Chin Hosp, 2020, 24(2): 8-11. DOI: 10.19660/j.issn.1671-0592.2020.02.03. Bian J, Wei LY, Shao XN, et al. Investigation and analysis of prescription review and evaluation in Chinese hospitals[J]. Chin Hosp, 2020, 24(2): 8-11. DOI: 10.19660/j.issn.1671-0592.2020.02.03.
5、林进方, 余剑波, 秦艳芳. 审方系统在提升我院门诊合理用药水平中的应用效果[J]. 中国当代医药, 2019, 26(10): 172-174.Lin JF, Yu JB, Qin YF. Application effect of prescription review system in promoting the level of rational drug use in outpatient department of our hospital[J]. China Mod Med, 2019, 26(10): 172-174. Lin JF, Yu JB, Qin YF. Application effect of prescription review system in promoting the level of rational drug use in outpatient department of our hospital[J]. China Mod Med, 2019, 26(10): 172-174.
6、杨彪. 处方前置审核系统在社区卫生服务中心处方管理中的应用效果分析[J]. 中国社区医师, 2025, 41(6): 165-167.Yang B. Analysis of the application effect of prescription pre-review system in prescription management of community health service centers[J]. Chin Community Dr, 2025, 41(6): 165-167. Yang B. Analysis of the application effect of prescription pre-review system in prescription management of community health service centers[J]. Chin Community Dr, 2025, 41(6): 165-167.
7、叶景乔. 处方前置审核对促进门诊合理用药的应用价值探讨[J]. 北方药学, 2021, 18(4): 101-103. DOI: 10.3969/j.issn.1672-8351.2021.04.052.Ye JQ. Discussion on the application value of prescription pre-audit in promoting rational drug use in outpatient department[J]. J N Pharm, 2021, 18(4): 101-103. DOI: 10.3969/j.issn.1672-8351.2021.04.052. Ye JQ. Discussion on the application value of prescription pre-audit in promoting rational drug use in outpatient department[J]. J N Pharm, 2021, 18(4): 101-103. DOI: 10.3969/j.issn.1672-8351.2021.04.052.
8、谢珊珊, 刘松, 万瑾瑾, 等. 药师借助合理用药软件开展门急诊处方前置审核的实践及效果[J]. 中国药房, 2021, 32(7): 876-880. DOI: 10.6039/j.issn.1001-0408.2021.07.18.Xie SS, Liu S, Wan JJ, et al. Practice and effect of outpatient and emergency prescription pre-audit by pharmacists with the help of rational drug use software[J]. China Pharm, 2021, 32(7): 876-880. DOI: 10.6039/j.issn.1001-0408.2021.07.18. Xie SS, Liu S, Wan JJ, et al. Practice and effect of outpatient and emergency prescription pre-audit by pharmacists with the help of rational drug use software[J]. China Pharm, 2021, 32(7): 876-880. DOI: 10.6039/j.issn.1001-0408.2021.07.18.
9、李姝姝,葛莉莉,闵琳,等.我院门诊实施处方前置审核的实践和效果[J].临床合理用药,2024,17(13):112-116.DOI:10.15887/j.cnki.13-1389/r.2024.13.032.Li SS, Ge LL, Min L, et al. The practice and effect of implementing pre-prescription review in our hospital's outpatient department. 2024,17(13):112-116.DOI:10.15887/j.cnki.13-1389/r.2024.13.032.Li SS, Ge LL, Min L, et al. The practice and effect of implementing pre-prescription review in our hospital's outpatient department. 2024,17(13):112-116.DOI:10.15887/j.cnki.13-1389/r.2024.13.032.
10、杜旭,刘玲.处方前置审核系统规则维护联合处方点评对急诊儿科处方质量的影响[J].临床合理用药,2023,16(11):44-47+51.DOI:10.15887/j.cnki.13-1389/r.2023.11.012.Du X, Liu L. Effect of prescription pre-audit system rules maintenance combined with prescription comments on the quality of emergency pediatric prescriptions[J]. Chinese Journal of Clinical Rational Drug Use. 2023,16(11):44-47+51.DOI:10.15887/j.cnki.13-1389/r.2023.11.012.Du X, Liu L. Effect of prescription pre-audit system rules maintenance combined with prescription comments on the quality of emergency pediatric prescriptions[J]. Chinese Journal of Clinical Rational Drug Use. 2023,16(11):44-47+51.DOI:10.15887/j.cnki.13-1389/r.2023.11.012.
11、刘慧,李婷.中药饮片前置审方规则的设置对处方合理率的提升作用[J].中国卫生产业,2025,22(06):55-57+65.DOI:10.16659/j.cnki.1672-5654.2025.06.055.Liu H, Li T. To Improve the Rational Rate of Prescription by Setting up the Pre-Review Rules of Chinese Herbal Pieces[J] China's health industry. 2025,22(06):55-57+65.DOI:10.16659/j.cnki.1672-5654.2025.06.055.Liu H, Li T. To Improve the Rational Rate of Prescription by Setting up the Pre-Review Rules of Chinese Herbal Pieces[J] China's health industry. 2025,22(06):55-57+65.DOI:10.16659/j.cnki.1672-5654.2025.06.055.
12、田晶, 曹国锋, 王婧, 等. 基于前置审方系统的药师干预审方体系促进门诊合理用药分析[J]. 临床合理用药, 2025, 18(6): 150-153, 157. DOI: 10.15887/j.cnki.13-1389/r.2025.06.041.
Tian J, Cao GF, Wang J, et al. Analysis of pharmacists' pre-trial prescription system based on pre-trial prescription system to promote rational drug use in outpatient department[J]. Chin J Clin Ration Drug Use, 2025, 18(6): 150-153, 157. DOI: 10.15887/j.cnki.13-1389/r.2025.06.041. Tian J, Cao GF, Wang J, et al. Analysis of pharmacists' pre-trial prescription system based on pre-trial prescription system to promote rational drug use in outpatient department[J]. Chin J Clin Ration Drug Use, 2025, 18(6): 150-153, 157. DOI: 10.15887/j.cnki.13-1389/r.2025.06.041.
13、付广俊, 张慧芝, 张颖. 二类精神药品处方前置审核规则库精细化设置与实践[J]. 中国药物滥用防治杂志, 2025, 31(1): 19-21, 28. DOI: 10.15900/j.cnki.zylf1995.2025.01.005.
Fu GJ, Zhang HZ, Zhang Y. Fine-grained setting and practice of pre-review rule database for prescription of second-class psychotropic drugs[J]. Chin J Drug Abuse Prev Treat, 2025, 31(1): 19-21, 28. DOI: 10.15900/j.cnki.zylf1995.2025.01.005. Fu GJ, Zhang HZ, Zhang Y. Fine-grained setting and practice of pre-review rule database for prescription of second-class psychotropic drugs[J]. Chin J Drug Abuse Prev Treat, 2025, 31(1): 19-21, 28. DOI: 10.15900/j.cnki.zylf1995.2025.01.005.
14、彭妞, 何杏仪, 欧阳燕婷, 等. 药师借助中药合理用药系统对门诊处方前置审核工作模式的实践与探讨[J]. 中外医药研究, 2024(29): 162-164. DOI: 10.3969/j.issn.2096-6229.2024.29.054.
Peng N, He XY, Ouyang YT, et al. Pharmacists' practice and discussion on the working mode of pre-examination of outpatient prescription with the help of rational drug use system of traditional Chinese medicine[J]. J Chin Foreign Med Pharm Res, 2024(29): 162-164. Peng N, He XY, Ouyang YT, et al. Pharmacists' practice and discussion on the working mode of pre-examination of outpatient prescription with the help of rational drug use system of traditional Chinese medicine[J]. J Chin Foreign Med Pharm Res, 2024(29): 162-164.
15、张怡丹, 申键, 王横溢. 处方前置审核系统对门诊处方合格率的影响[J]. 实用医技杂志, 2024, 31(10): 752-755. DOI: 10.19522/j.cnki.1671-5098.2024.10.018.
Zhang YD, Shen J, Wang HY. The impact of prescription pre-review system on the qualification rate of outpatient prescriptions[J]. J Pract Med Tech, 2024, 31(10): 752-755. DOI: 10.19522/j.cnki.1671-5098.2024.10.018. Zhang YD, Shen J, Wang HY. The impact of prescription pre-review system on the qualification rate of outpatient prescriptions[J]. J Pract Med Tech, 2024, 31(10): 752-755. DOI: 10.19522/j.cnki.1671-5098.2024.10.018.
16、吴凯珊, 伍俊妍, 郑志华, 等. 超说明书用药的处方审核基本要素与方法[C]//2021第26届广东省药师周大会论文集. 广州, 2020: 100-114.
Wu KS, Wu JY, Zheng ZH, et al. Key element and methods on prescription review of off-label use [C]. Proceedings of the 26th Guangdong Province Pharmacist Week Conference in 2021, Guangzhou, 2020: 100-114. DOI: 10.26914/c.cnkihy.2020.074290. Wu KS, Wu JY, Zheng ZH, et al. Key element and methods on prescription review of off-label use [C]. Proceedings of the 26th Guangdong Province Pharmacist Week Conference in 2021, Guangzhou, 2020: 100-114. DOI: 10.26914/c.cnkihy.2020.074290.
17、马靖, 李晨. 基于循证证据的睡眠障碍超说明书用药处方前置审核[J]. 中国药房, 2022, 33(19): 2414-2417+2427. DOI: 10.6039/j.issn.1001-0408.2022.19.22.Ma J, Li C. Evidence-based pre-prescribing review to standardize off-label drug use for sleep disorders[J]. China Pharm, 2022, 33(19): 2414-2417+2427. DOI: 10.6039/j.issn.1001-0408.2022.19.22. Ma J, Li C. Evidence-based pre-prescribing review to standardize off-label drug use for sleep disorders[J]. China Pharm, 2022, 33(19): 2414-2417+2427. DOI: 10.6039/j.issn.1001-0408.2022.19.22.
18、潘莹, 魏雪, 刘澍, 等. 基于信息系统探索肿瘤专科超说明书用药管理[J]. 今日药学, 2025, 35(2): 114-117.Pan Y, Wei X, Liu S, et al. Exploring management of oncological off-label use based on information system[J]. Pharm Today, 2025, 35(2): 114-117. an Y, Wei X, Liu S, et al. Exploring management of oncological off-label use based on information system[J]. Pharm Today, 2025, 35(2): 114-117.
19、中华医学会皮肤性病学分会荨麻疹研究中心, 徐金华, 郝飞, 等. 中国荨麻疹诊疗指南(2022版)[J]. 中华皮肤科杂志, 2022, 55(12): 1041-1049. DOI: 10.35541/cjd.20220609. Centre for Urticaria Research, Chinese Society of Dermatology, Xu JH, Hao F, et al. Guideline for diagnosis and treatment of urticaria in China(2022)[J]. Chin J Dermatol, 2022, 55(12): 1041-1049. DOI: 10.35541/cjd.20220609.Centre for Urticaria Research, Chinese Society of Dermatology, Xu JH, Hao F, et al. Guideline for diagnosis and treatment of urticaria in China(2022)[J]. Chin J Dermatol, 2022, 55(12): 1041-1049. DOI: 10.35541/cjd.20220609.
20、关于调整省直城镇职工医疗保障相关政策的通知[EB/OL]. (2020-06-30). http://ybj.jl.gov.cn/zcfg/sjzc/202006/t20200630_7294401.html.
Notice on Adjusting Relevant Policies for Medical Security of Urban Employees in Provincial Government Agencies[EB/OL]. (2020-06-30). http://ybj.jl.gov.cn/zcfg/sjzc/202006/t20200630_7294401.html.Notice on Adjusting Relevant Policies for Medical Security of Urban Employees in Provincial Government Agencies[EB/OL]. (2020-06-30). http://ybj.jl.gov.cn/zcfg/sjzc/202006/t20200630_7294401.html.
21、国家卫生健康委办公厅 国家医保局办公室关于印发长期处方管理规范(试行)的通知[EB/OL]. (2021-08-13). https://www.nhsa.gov.cn/art/2021/8/13/art_104_6538.html.
Notice of the Office of the National Medical Insurance Administration of the General Office of the National Health Commission on the issuance of Regulations for long-term Prescription management (Trial implementation) [EB/OL]. (2021-08-13). https://www.nhsa.gov.cn/art/2021/8/13/art_104_6538.html.Notice of the Office of the National Medical Insurance Administration of the General Office of the National Health Commission on the issuance of Regulations for long-term Prescription management (Trial implementation) [EB/OL]. (2021-08-13). https://www.nhsa.gov.cn/art/2021/8/13/art_104_6538.html.
22、王持, 李雪芹, 刘锐锋, 等. 住院医嘱前置审方异步模式的构建与实践效果[J]. 中国药业, 2024, 33(23): 26-30. DOI: 10.3969/j.issn.1006-4931.2024.23.006.
Wang C, Li XQ, Liu RF, et al. Construction of an asynchronous mode of inpatient-order pre-prescription review and its application effect[J]. China Pharm, 2024, 33(23): 26-30. DOI: 10.3969/j.issn.1006-4931.2024.23.006. Wang C, Li XQ, Liu RF, et al. Construction of an asynchronous mode of inpatient-order pre-prescription review and its application effect[J]. China Pharm, 2024, 33(23): 26-30. DOI: 10.3969/j.issn.1006-4931.2024.23.006.
23、陈宝燕. 基于信息化技术与多源证据的精细化医嘱审核实践[J]. 中国现代应用药学, 2024, 41(16): 2283-2287. DOI: 10.13748/j.cnki.issn1007-7693.20230568.
Chen BY. Practice of information technology and multi-source evidence refined medical order review[J]. Chin J Mod Appl Pharm, 2024, 41(16): 2283-2287. DOI: 10.13748/j.cnki.issn1007-7693.20230568. Chen BY. Practice of information technology and multi-source evidence refined medical order review[J]. Chin J Mod Appl Pharm, 2024, 41(16): 2283-2287. DOI: 10.13748/j.cnki.issn1007-7693.20230568.
24、岳卫琴, 邹奇锋, 曾海. “审方系统+临床药师” 的审核模式对PIVAS不合理医嘱干预的效果分析[J]. 北方药学, 2024, 21(7): 87-90. DOI: 10.3969/j.issn.1672-8351.2024.07.029.
Yue WQ, Zou QF, Zeng H. Analysis of the effect of the audit mode of “prescription system+clinical pharmacist” on unreasonable doctor’s advice intervention of PIVAS[J]. J N Pharm, 2024, 21(7): 87-90. DOI: 10.3969/j.issn.1672-8351.2024.07.029. Yue WQ, Zou QF, Zeng H. Analysis of the effect of the audit mode of “prescription system+clinical pharmacist” on unreasonable doctor’s advice intervention of PIVAS[J]. J N Pharm, 2024, 21(7): 87-90. DOI: 10.3969/j.issn.1672-8351.2024.07.029.
25、高艳斓, 魏丹丹. 处方前置审核系统对静脉用药调配中心不合理医嘱的干预效果[J]. 临床合理用药, 2024, 17(23): 150-153. DOI: 10.15887/j.cnki.13-1389/r.2024.23.041.
Gao YL, Wei DD. Intervention effect of prescription pre-audit system on unreasonable doctor’s orders in intravenous drug dispensing center[J]. Chin J Clin Ration Drug Use, 2024, 17(23): 150-153. DOI: 10.15887/j.cnki.13-1389/r.2024.23.041. Gao YL, Wei DD. Intervention effect of prescription pre-audit system on unreasonable doctor’s orders in intravenous drug dispensing center[J]. Chin J Clin Ration Drug Use, 2024, 17(23): 150-153. DOI: 10.15887/j.cnki.13-1389/r.2024.23.041.
26、Personalized Drug Therapy Key Laboratory of Sichuan Province, Department of Pharmacy, Sichuan Academy of Medical Sciences, 等. 国家重点监控药品超说明书临床合理应用专家共识[J]. 医药导报, 2023, 42(12): 1737-1751.Personalized Drug Therapy Key Laboratory of Sichuan Province, Pharmacy DO, Sichuan Academy of Medical Sciences, et al. National key monitoring drugs(the second batch) expert consensus on rational clinical application for off-label drug uses[J]. Her Med, 2023, 42(12): 1737-1751. Personalized Drug Therapy Key Laboratory of Sichuan Province, Pharmacy DO, Sichuan Academy of Medical Sciences, et al. National key monitoring drugs(the second batch) expert consensus on rational clinical application for off-label drug uses[J]. Her Med, 2023, 42(12): 1737-1751.
27、边原, 陈岷, 杜姗, 等. 第二批国家重点监控药品合理使用规范[J]. 中国药房, 2023, 34(20): 2433-2453. DOI: 10.6039/j.issn.1001-0408.2023.20.01.Bian Y, Chen M, Du S, et al. Principles for the rational use of national key monitoring drugs(the second batch)[J]. China Pharm, 2023, 34(20): 2433-2453. DOI: 10.6039/j.issn.1001-0408.2023.20.01.Bian Y, Chen M, Du S, et al. Principles for the rational use of national key monitoring drugs(the second batch)[J]. China Pharm, 2023, 34(20): 2433-2453. DOI: 10.6039/j.issn.1001-0408.2023.20.01.
28、田媛媛, 张丽. 处方前置审核助力PDCA模式下骨科中成药的合理应用探索[J]. 医药论坛杂志, 2025, 46(1): 89-94. DOI: 10.20159/j.cnki.jmf.2025.01.019.Tian YY, Zhang L. Research on improvement of rational application of Chinese patent medicines in orthopedics under PDCA model with support of pre-prescription review[J]. J Med Forum, 2025, 46(1): 89-94. DOI: 10.20159/j.cnki.jmf.2025.01.019. Tian YY, Zhang L. Research on improvement of rational application of Chinese patent medicines in orthopedics under PDCA model with support of pre-prescription review[J]. J Med Forum, 2025, 46(1): 89-94. DOI: 10.20159/j.cnki.jmf.2025.01.019.