Diabetic retinopathy (DR) is a leading cause of blindness that affects millions of people in the U.S. (1), with the prevalence of DR and vision-threatening retinopathy among patients with diabetes estimated to be 28–40% and 4–8%, respectively (2,3). In addition to the devastating consequences on those with the disease, the care of DR has been estimated to cost the U.S. healthcare system $500 million annually, placing a significant economic burden (4). Fortunately, early detection and treatment have shown to significantly improve clinical and cost-effectiveness (5,6).
As a result, much emphasis has been placed on developing effective and accessible ways of screening for DR and other retinal pathologies. Screening for DR has clear guidelines (7-9) and is becoming increasingly mandated in the U.S. Regardless, screening via traditional methods (e.g., dilated fundus exam by eye care specialists) is heavily dependent on resource availability in each community and thus frequently is not very successful (10-14). Teleophthalmology has attempted to address this unmet need, and while it can be very effective, there are yet several barriers to its widespread implementation, including cost and lack of universal standards (15,16). While there are many approaches to teleophthalmology, one type hereby referred to as remote diagnosis, has been proposed as a particularly effective form of screening (17). In contrast to other forms of teleophthalmology screening, remote diagnosis uses imaging devices permanently located at the point of service (e.g., primary care or endocrinology clinics) and operated by non-expert imagers (e.g., office clinical medical assistants, CMAs). Thus, it holds the potential to be more accessible and cost-effective.
The feasibility of such a model has recently been successfully tested in the detection of referable DR and age-related macular degeneration (AMD), using fundus photography and optical coherence tomography (OCT) camera located at the point of service such as endocrinology clinics and assisted living centers (17). This study showed near equivalent rates of detection compared to standard examination by a retinal specialist (17). Other imaging methods such as ultra-wide-field scanning laser ophthalmoscopy (Optomap), which are possibly better suited for imaging of the fundus through non-dilated pupils, have yet to be explored with a remote diagnosis model. However, these methods have significant costs associated with an expert grader required to review imaging findings and decide whether to refer the patient for further examination. Additionally, grading and information transfer are associated with a significant delay in decision making and patient scheduling. One way of bypassing the need for an expert grader is to train and implement a deep learning (DL) model to automate the grading. Previous studies have trained DL models using Optomap images to detect AMD (18), central retinal vein occlusion (19), macular holes (20), retinal detachment (21), and DR (22,23). However, none of these studies used Optomap images acquired in a prospective manner, in a remote diagnosis approach to detect referable retinal pathologies, including DR.
Thus, the purposes of the present study are twofold. First, we evaluate the feasibility of a remote diagnosis approach to screen for DR and other referable retinal pathology at two Duke endocrinology clinics using the UWF Primary imaging device by Optos. Then, we introduce a DL model using the acquired Optomap images and test its diagnostic precision compared to clinician evaluation of the same images as the reference standard. We present the following article in accordance with the STARD reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-21-53/rc).
This prospective, nonrandomized study was conducted in accordance with the tenets of the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Duke University (IRB00012400) and informed consent was taken from all individual participants.
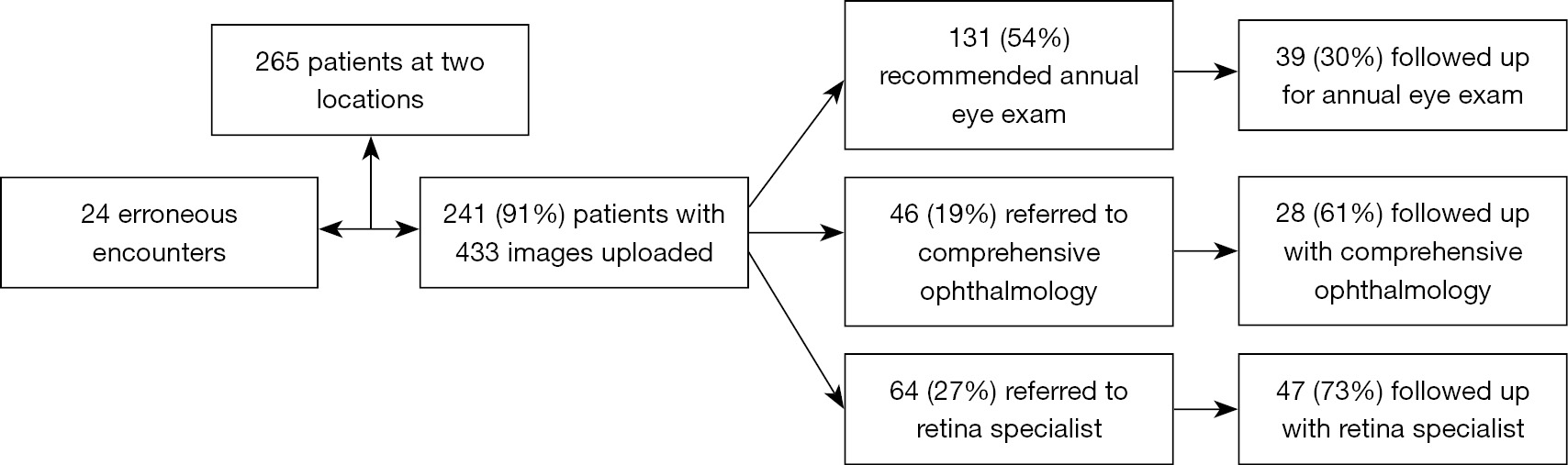
A total of 265 patients were enrolled from 2 Duke University Heath System endocrinology clinics. All eligible patients over the age of 18 seen at these outpatient sites were invited to participate in this study. Pregnant or nursing women were excluded, as well as any patients who could not sit still for the duration of the imaging or tolerate the imaging. Patients were informed of risks and benefits prior to study involvement, and consent was obtained from all patients.
Remote diagnostic imaging of non-dilated pupils was performed in the endocrinology clinics by trained but non-expert imagers provided by the clinics. Non-ophthalmologic health care professionals (e.g., clinical medical assistants, CMAs) were trained to use an FDA-approved noncontact, portable retinal imaging device (Optomap, Nikon) that takes up to 200o field-of-view retinal images. After the initial training, imagers were observed and coached periodically to ensure that the imaging remained at a high quality.
A large percentage of the screened patients referred for further evaluation followed up with either Duke University Health System retinal specialists or comprehensive ophthalmologists for standard examination, including a dilated fundus exam and any ancillary testing (e.g., color fundus photo, optical coherence tomography, fundus angiography) performed by expert photographers. Thus, each eye served as its own control for those patients. Figure 1 depicts a flowchart of our study. At the conclusion of the study, the non-ophthalmologic health care professionals trained to use the Optomap imaging device completed a non-validated survey to assess the ease of using the device and acquiring images.
Two graders (M.H. and D.B.) graded the images by consensus, without any access to the patients’ clinical information. All images were graded by the end of the work week during which they were acquired. Images were considered ungradable if no clear view of the macula was available (e.g., poor positioning, poor patient compliance, movement, droopy eyelids). Images were assessed for DR and its severity using the standardized ETDRS scale. By default, patients with DR on remote diagnosis imaging were referred to a retina specialist. Other findings were referred either to a retina specialist or comprehensive ophthalmology, depending on apparent severity. Any ungradable images were grouped with those with referable disease, as these eyes would have failed image screening. For accuracy assessment, the reference standard was the standard clinical examination findings from the same patient.
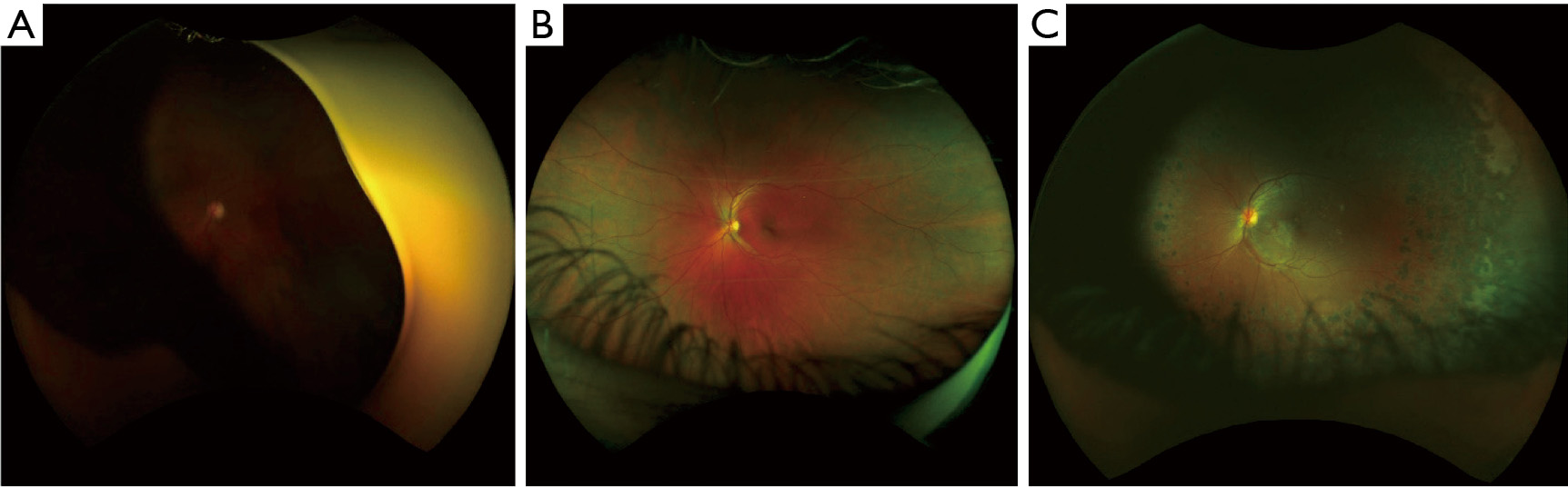
A total of 433 3-channel Optomap images acquired during the study were used to train and test our DL model. Of all these images, 269 were labeled as normal according to remote diagnosis evaluation, and the remaining 164 were labeled as having some retinal pathology, whether DR or other pathologies. Figure 2 shows 3 representative examples of Optomap images: ungradable, normal, and diagnosed with DR.
We adopted a 4:1 ratio for splitting all the images into training and testing sets. To increase the amount of data and improve the robustness of the model, the images in the training dataset underwent augmentation. The augmentation operations involved: (I) cropping 0 to 16 pixels from each side of the images randomly; (II) flipping the images horizontally or vertically; (III) rotating the images between negative 25 degrees to positive 25 degrees. The purposes of augmentation were to increase the number of training data to reduce overfitting and improve the generalizability of our model. The images in the testing set remain unchanged. Figure 3 shows a flow chart of our proposed DL approach.
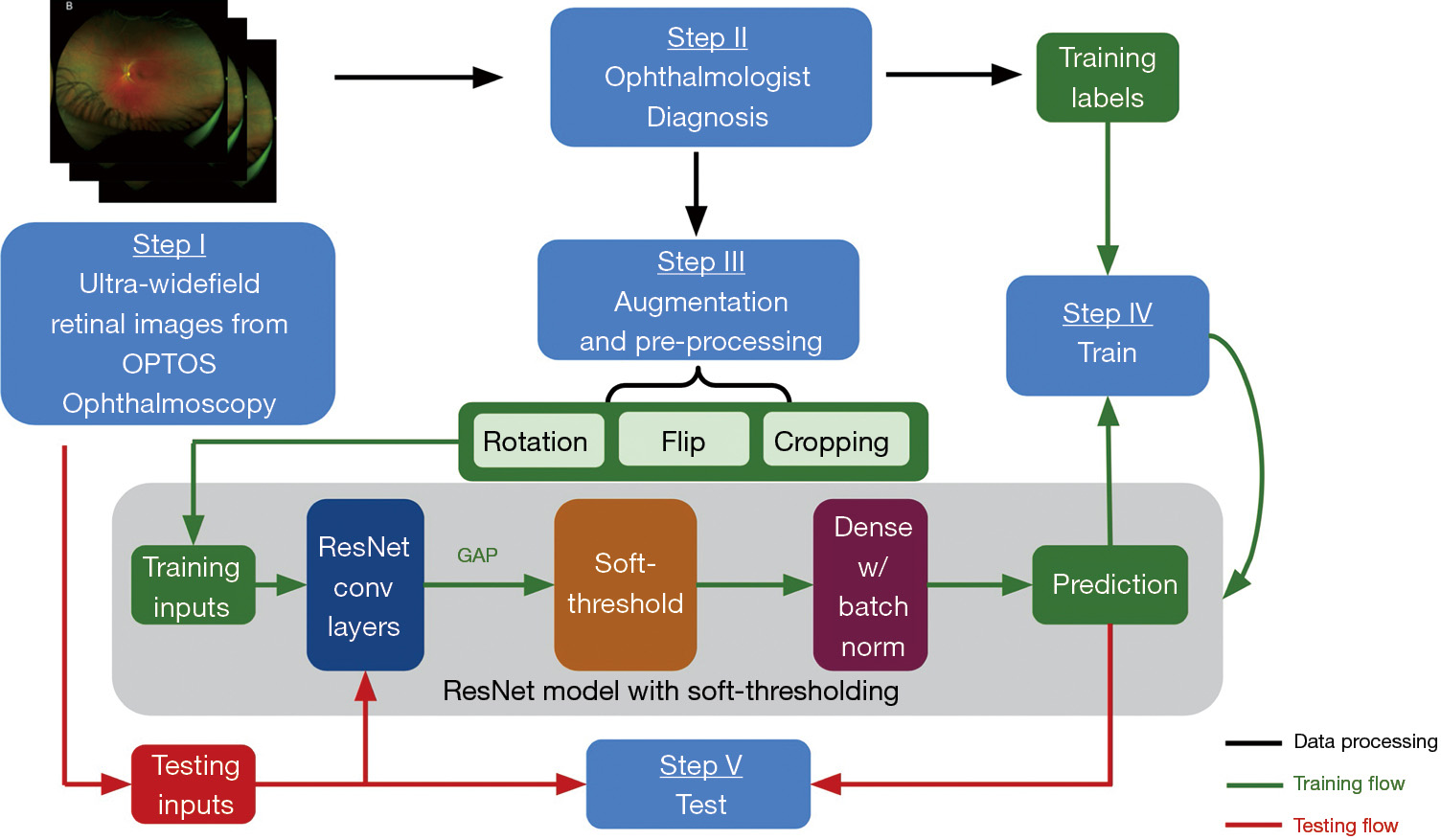
A DL model was built from scratch using the Keras library in Python to identify the presence or absence of retinal pathologies on Optomap images, using a convolutional neural network (CNN) model built on top of ResNet’s (24) architecture. We did not employ transfer learning since the standard well-established models are pre-trained on public datasets like ImageNet, which contains substantially different images from our UWF Optomap images. Besides, since large databases of ultrawide field images are not as readily available (compared to simple fundus photos, for example), there were not enough images to pre-train the model. Thus, we followed the classical architecture of ResNet to build a small model and additionally added soft-thresholding techniques to reduce the noise.
Specifically, ResNet is a CNN that uses shortcuts to bypass some layers to alleviate the vanishing gradient problem caused by increasing depths in deep neural networks. However, it falls short in analyzing images that are highly noisy or include substantial artifacts (24). As a result, the eyebrows and eyelids presented in the Optomap images could prevent ResNet models from classifying the images accurately. Instead, as proposed by Zhao et al. (24), we applied a trainable soft threshold to the features outputted from the global average pooling (GAP) layer. This helps the CNN to focus on the features that are most helpful for improving its performance during training as well as disregarding the noisy information introduced by the artifacts from the input images. Similar techniques are widely used for other denoising methods (25,26). Specifically, as shown in Figure 3, soft-thresholding layer was employed after the GAP layer, followed by dense layers with rectified linear unit (27) as the activation function and batch normalization. The batch normalization standardized the inputs to the dense layer and improved the training speed and stability (28). Finally, the binary classification was implemented by the dense layer after global average pooling.
The images were divided into mini batches of size 200, and the model was trained for 5,000 epochs. The Adam optimizer (29) was chosen as the optimization algorithm, and the learning rate was 0.001. Early stopping—which monitored the validation loss—was set, and the minimum delta and patience were 0 and 2,000, respectively. This ensured that if the model’s validation loss did not decrease within 2,000 epochs, the training would be stopped. The model was enabled to restore weights from the epoch that achieved minimum validation loss. Training accuracy curves, training loss curves, validation accuracy curves, and validation loss curves were recorded to fine-tune the parameters. This study’s hardware specification was an Intel(R) Xeon(R) CPU @ 2.20GHz CPU, a Tesla P100 GPU, and 13GB memory. The code we used to train the CNN has been made open source on GitHub (28).
All statistical analyses were performed with open-source software (Python, version 3.7.6; Python Software Foundation, https://www.python.org). Descriptive statistics were calculated to assess the baseline demographics and medical comorbidities. Chi-square test was used to assess the association of categorical variables, two-sample t-tests were used to test differences in means, and logistic regressions were used to assess the relationship between predictor variables and a binary outcome. Cohen’s kappa was used to test agreement between remote diagnosis and standard examination by eye specialists at a tertiary care center. Per standards set by Landis and Koch (30), a kappa of 0.00–0.20 indicated slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.00, almost perfect agreement. Ninety-five percent confidence intervals were derived by bootstrapping with 1,000 replications. To assess the performance of our DL model, we calculated sensitivity, specificity, and the area under the receiver operating characteristic curve (AUROC), along with each metric’s respective 95% confidence intervals. The alpha level (type I error) was set at 0.05 for all statistical analyses.
A total of 265 patients agreed to participate. However, due to various technical or logistical difficulties (e.g., patient positioning), 433 eyes of 241 patients were imaged. The mean age was 50±17 years, and 45% of patients were female. Of all patients, 56% identified as white, 31% as black or African American, 6% as Asian, 2% as multiracial, and 5% did not disclose or identify as any of the above. Thirty-four percent of the patients had a diagnosis of diabetes mellitus type 1, and the other 66% had a diagnosis of diabetes mellitus type 2. The average Hemoglobin A1c was 8.8%±2.3% (normal range, 5.7–6.4%), and 81% of the patients were on Insulin. Table 1 lists baseline demographic information as well as the most common medical comorbidities for the overall cohort and subgroups stratified by type of diabetes (type 1 or type 2). While patients with Type 2 diabetes mellitus (T2DM) were more likely than Type 1 diabetic patients (T1DM) to have several comorbidities including hypertension (P<0.001), hyperlipidemia (P<0.001), and chronic kidney disease (P=0.027), among others, they had comparable rates of DR (P=0.741) and rates of referral to a retina specialist (P=0.725) or comprehensive ophthalmologist (P=0.657).
| Variables | All, n=265 | T1DM, n=86? | T2DM, n=168? | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean ± SD, years | 50.1±16.7 | 34.3±13.8 | 57.8±12.2 | <0.001a |
| Sex, female | 45% | 45% | 45% | 0.979b |
| Race | <0.001b | |||
| Caucasian | 56% | 79% | 45% | |
| African American | 31% | 17% | 38% | |
| Hemoglobin A1c, mean ± SD | 8.8±2.3 | 8.3±1.9 | 9.0±2.5 | 0.011a |
| Duration of diabetes, mean ± SD, years | 3.6±3.4 | 3.2±3.4 | 3.8±3.3 | 0.163a |
| Insulin use | 81% | 100% | 72% | <0.001b |
| Comorbidities | ||||
| Hypertension | 51% | 22% | 65% | <0.001b |
| Obesity | 46% | 27% | 58% | <0.001b |
| Hyperlipidemia | 28% | 7% | 39% | <0.001b |
| OSA | 13% | 4% | 19% | 0.001b |
| GERD | 13% | 2% | 19% | <0.001b |
| OA | 12% | 4% | 17% | 0.005b |
| Coronary artery disease | 11% | 5% | 14% | 0.035b |
| Anemia | 11% | 2% | 14% | 0.006b |
| Chronic kidney disease | 9% | 4% | 13% | 0.027b |
| Remote diagnosis evaluation | ||||
| Diabetic retinopathy | 22% | 24% | 21% | 0.741b |
| Referral to retina specialist | 27% | 25% | 28% | 0.725b |
| Referral to comprehensive ophthalmologist | 19% | 16% | 19% | 0.657b |
a, compared using two-sample t-test; b, compared using chi-square test; ?, type of diabetes was unknown in 11 patients. GERD, gastroesophageal reflux disease; OA, osteoarthritis; OSA, obstructive sleep apnea; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
After remote evaluation of Optomap images by retina specialists, as expected, a large proportion of screened patients (54%) had no retinal pathology identified and were advised to follow-up annually for DR screening. Sixty-four patients (27%) received a referral to a retina specialist for further assessment and treatment, and 46 patients (19%) received a referral to a comprehensive ophthalmology for management of incidental findings. Of those patients referred for further ophthalmic attention, 52 had DR. Among these 52 patients, 32% had mild, 46% moderate, 10% severe non-proliferative DR, and 12% had proliferative DR. The most common incidental findings in eyes referred to a retinal specialist was AMD, and the most common incidental findings in those referred to a comprehensive ophthalmologist were choroidal nevi and pigmentary changes.
Using logistic regression modeling, we evaluated several factors that affected the likelihood of DR (Table 2) and retinal referral (Table 3) in remote diagnosis evaluation. Patients with greater HbA1c were more likely to have DR on remote evaluation [odds ratio (OR) 1.16 for each 1% increase in HbA1c, 95% CI: 1.02–1.33, P=0.029] as did patients on insulin (OR 3.62, 95% CI: 1.23–10.63, P=0.019). Patients with chronic kidney disease were also more likely to have DR (OR 2.96, 95% CI: 1.23–7.12, P=0.016). Obesity (OR 1.81, 95% CI: 1.01–3.24, P=0.045) and congestive heart failure (OR 7.54, 95% CI: 1.42–39.93, P=0.017) were associated with referral to a retinal specialist.
| Predictor | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Demographics | |||
| Age | 1.01 | [0.99, 1.03] | 0.429 |
| Sex, male | 1.13 | [0.60, 2.09] | 0.709 |
| Race, Caucasian | 0.73 | [0.38, 1.43] | 0.365 |
| Hemoglobin A1c | 1.16 | [1.02, 1.33] | 0.029 |
| Duration of diabetes | 0.94 | [0.85, 1.05] | 0.277 |
| Insulin use | 3.62 | [1.23, 10.63] | 0.019 |
| Comorbidities | |||
| Hypertension | 1.24 | [0.67, 2.30] | 0.488 |
| Hyperlipidemia | 0.55 | [0.26, 1.17] | 0.118 |
| Obesity | 1.48 | [0.80, 2.73] | 0.216 |
| OSA | 0.66 | [0.24, 1.82] | 0.425 |
| Coronary artery disease | 1.16 | [0.44, 3.07] | 0.765 |
| Chronic kidney disease | 2.96 | [1.23, 7.12] | 0.016 |
| GERD | 0.63 | [0.23, 1.74] | 0.376 |
| Congestive heart failure | 1.46 | [0.28, 7.77] | 0.654 |
| Hypothyroidism | 2.10 | [0.74, 5.98] | 0.165 |
OSA, obstructive sleep apnea; GERD, gastroesophageal reflux disease.
| Predictor | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Demographics | |||
| Age | 1.02 | [1.00, 1.04] | 0.056 |
| Sex, male | 1.10 | [0.62, 1.96] | 0.748 |
| Race, Caucasian | 0.55 | [0.29, 1.02] | 0.059 |
| Hemoglobin A1c | 1.20 | [1.05, 1.37] | 0.006 |
| Duration of diabetes | 1.04 | [0.95, 1.13] | 0.379 |
| Insulin use | 2.38 | [1.00, 5.64] | 0.050 |
| Comorbidities | |||
| Hypertension | 1.65 | [0.92, 2.95] | 0.092 |
| Hyperlipidemia | 0.94 | [0.49, 1.79] | 0.848 |
| Obesity | 1.81 | [1.01, 3.24] | 0.045 |
| OSA | 0.80 | [0.33, 1.95] | 0.619 |
| Coronary artery disease | 1.68 | [0.70, 4.01] | 0.246 |
| Chronic kidney disease | 2.20 | [0.92, 5.24] | 0.076 |
| GERD | 1.33 | [0.59, 2.99] | 0.491 |
| Congestive heart failure | 7.54 | [1.42, 39.93] | 0.017 |
| Hypothyroidism | 2.09 | [0.76, 5.74] | 0.154 |
OSA, obstructive sleep apnea; GERD, gastroesophageal reflux disease.
Of the 433 images, 404 (93%) were deemed gradable. A total of 114 patients out of the 241 patients that were imaged during remote diagnoses followed up at Duke Eye Center for a standard examination by a retinal specialist (breakdown by type of referral shown in Figure 1). Cohen’s kappa score for agreement between remote evaluation and standard examination was 0.58 (95% CI: 0.44–0.72), which indicated moderate agreement. There were 13 eyes in which remote diagnosis with Optomap images missed a retinal pathology and 11 eyes with misdiagnoses compared to standard evaluation. These findings are summarized in Table 4.
| False-positive findings by remote diagnosis? (n=11) | Missed by remote diagnosis? (n=13) |
|---|---|
| Mild to moderate NPDR (n=8) | PDR (n=1) |
| Venous tortuosity (n=1) | Severe NPDR (n=2) |
| Macular lesions (n=1) | Mild to moderate NPDR (n=6) |
| Possible retinoschisis in the periphery (n=1) | Cystoid macular edema (n=1) |
| Dry AMD (n=3) |
?, indicates retinal findings noted on remote diagnosis evaluation but were not found on standard evaluation by a retinal specialist; ?, indicates retinal findings that were missed on remote diagnosis evaluation but were diagnosed on standard evaluation by a retinal specialist. NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; AMD, age-related macular degeneration.
On the non-validated survey of CMAs to assess the feasibility of implementing remote imaging and diagnosis as a standard practice, the results indicated that generally, the camera was well received, with about 75% of those surveyed expressing mastery with the camera. However, the results of our survey also indicated that about 50% of respondents had some difficulty correctly positioning patients to get the retina imaged as clearly as possible with the Optomap. Additionally, our survey results showed that, on average, it only required 3–6 minutes to image a patient’s retina.
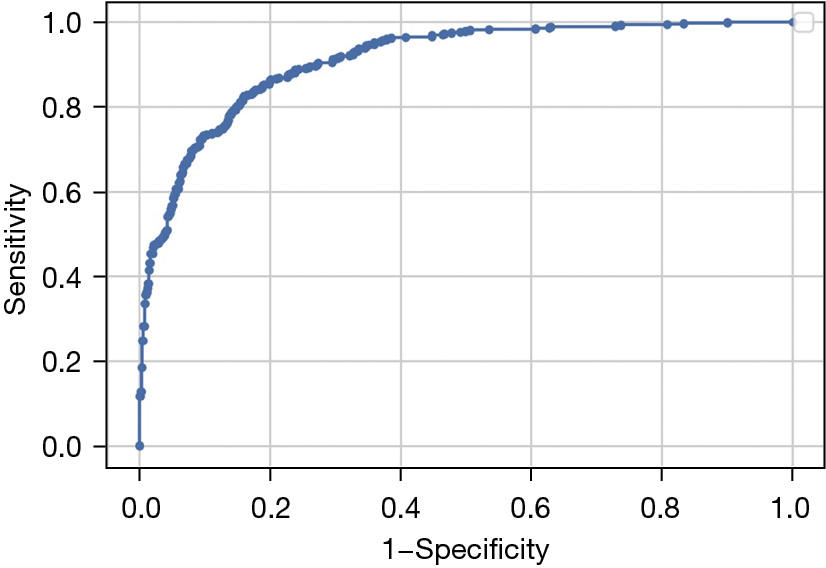
The accuracy of our DL model for identifying referable retinal pathology was 82.8% (95% CI: 80.3–85.2%), sensitivity was 81.0% (95% CI: 78.5–83.6%), and specificity was 73.5% (95% CI: 70.6–76.3%). Figure 4 shows the receiver operating characteristic (ROC) curve of our model for detecting referable retinal pathology from Optomap. The AUROC was 81.0% (95% CI: 78.5–83.6%). Table 5 summarizes additional performance metrics for our DL model.
| Metric | Performance |
|---|---|
| Accuracy | 82.8% (95% CI: 80.3–85.2%) |
| False negative rate | 19.0% (95% CI: 16.4–21.5%) |
| Sensitivity | 81.0% (95% CI: 78.5–83.6%) |
| Specificity | 73.5% (95% CI: 70.6–76.3%) |
| Positive predictive value | 81.4% (95% CI: 79.0–84.0%) |
| F1 score | 81.4% (95% CI: 78.9–83.9%) |
| AUROC | 81.0% (95% CI: 78.5–83.6%) |
AUROC, area under the receiver operating characteristics curve.
Burdened with multiple co-morbidities, diabetic patients seem to be very compliant on average with their primary care and endocrinology visits (31,32), making those clinics an ideal location to implement screening for DR. Herein, we demonstrate the feasibility of such a screening approach. We also demonstrate that the UWF camera Primary by Optos may be a good choice for a screening device at the point of service. To facilitate timely image interpretation and information transfer, we developed and made open-source an automated DL-based image interpretation model with a binary classification outcome (Yes/No retinal pathology). With a larger number of images, it is expected that the accuracy of the proposed DL model could be further improved. However, it is important to emphasize that, while previous studies have developed DL models to detect or stage DR and other retinal pathologies, this is the first computational model for automated analysis for identification of any referable pathology using Optomap images acquired in a prospective manner at multiple endocrinology clinics.
Retina screening in this setting has multiple advantages: improved accessibility, patient capture and triage, and clinical and cost-effectiveness. In some forms of teleophthalmology screening, color fundus photographs (CFP) are taken by expert photographers in mobile vans or dedicated brick-and-mortar imaging centers, then transmitted to grading experts. These approaches often suffer from high costs, requiring dedicated vehicles or office space, as well as photographers and graders. While CFP is the current standard in teleophthalmology care of retinal disease (7,9,33), we employed an UWF Optomap camera. Despite images being taken on non-dilated pupils by non-expert imagers, we found a relatively low percentage of ungradable images (15%) in our study. Our rate of ungradable images was higher than the rate of 6.1% previously published (11) which might be secondary to patient demographic factors (e.g., age, difficult positioning), different UWF imaging devices (Primary vs. Daytona), imager skill, or applied protocol (e.g., not to disturb clinic flow, we did not encourage our imagers to retake substandard images).
Our study demonstrated that in an environment with relatively high disease prevalence, 46% of screened patients required further ophthalmology attention. Out of all referred patients, 22% had some stage of DR and by consensus were referred to a retina specialist, 5% had retina-referable incidental finings (most commonly age-related macular degeneration, data not shown), and 19% had incidental findings referable to comprehensive ophthalmology (most commonly choroidal nevi and pigmentary changes, data not shown). We found that various factors were associated with an increased likelihood of being referred to a retina specialist. We found that higher HbA1c, chronic kidney disease, and obesity increased that likelihood. While this study was conducted in a population of relatively high disease prevalence (endocrinology clinic patient population), if this approach is used in a population of low disease prevalence, we would set different thresholds of various demographic and clinical characteristics (e.g., HbA1c levels) to potentially determine whom to screen with imaging. This would enrich the pre-test probability of disease in the screened population, thereby increasing the post-test probability, minimizing the false positive rate for referrals, and making the program more cost-effective and available to wider range of healthcare providers.
To potentially decrease the time and cost associated with image grading and information transfer, we developed a DL model to identify the presence of retinal pathology from acquired images. Our model achieved a sensitivity of 81%, specificity of 73%, and an AUROC of 81%. The combination of remote diagnosis with an accurate DL model would, in theory, bypass the need for expert graders to determine referable retinal pathologies and further improve the cost-effectiveness of screening, effectively facilitating the delivery of the grand promise of teleophthalmology.
Our remote diagnosis approach using Optomap images had a moderate agreement with a standard clinical evaluation by a retinal specialist or comprehensive ophthalmologist, yielding a Cohen’s kappa of 0.58. This was lower than our previous work (17) using an imaging device combining CFP and OCT, which achieved almost perfect agreement (Cohen’s kappa >0.80). The reason may be that many patients concurrently underwent the screening and standard examination in the prior study. Anyhow, this finding elucidates the need for further investigations on what might be the most appropriate imagining modality in a teleophthalmology setting. Multiple components are important for the efficient logistics behind successful DR telemedicine program, and the screening system is the critical one. The screening unit besides providing good image quality on the non-mydriatic pupil, has to also be cost-effective, of adequate size, and easy to use with a short time for image acquisition.
Our study has some limitations. The screened population was homogenous, consisting of diabetic patients seen at an endocrinology clinic. As such, the baseline prevalence of DR was relatively high. The performance of this approach in a population with relatively low disease prevalence (e.g., primary care clinics) needs to be validated. Additionally, we did not assess for retinal lesion distribution relative to the standard ETDRS photographic field. This might be needed to better understand the retinal area of interest for capture and whether using UWF retinal imaging vs. a narrow field camera combined with OCT would be more appropriate in this setting. Another limitation of this study that likely influenced Cohen’s agreement was that not all screened patients had standard examination following the screening. We believe that this is not always feasible and, in essence, defeats the purpose of a screening program, especially when thousands of patients get screened. Nonetheless, increasing the number of these patients would likely increase our study’s agreement outcome. Furthermore, while our DL model was trained simply to binarily classify Optomap images as the presence or absence of retinal pathology, future studies with larger datasets could develop models that more precisely classify retinal pathologies. For example, multilabel convolutional neural network (34) can be used to classify images as age-related macular degeneration, hypertensive retinopathy, or DR (and its stages).
Herein we demonstrate the feasibility of the remote diagnosis approach using Optos device Primary, combined with an automated DL model to identify referable retinal pathology. Emerging new imaging technologies with simplified interfaces that can be used in non-ophthalmic clinics by non-expert imagers, in conjunction with automated image interpretation, make remote diagnosis very attractive to take the lead in early diagnosis and monitoring of retinal pathology.