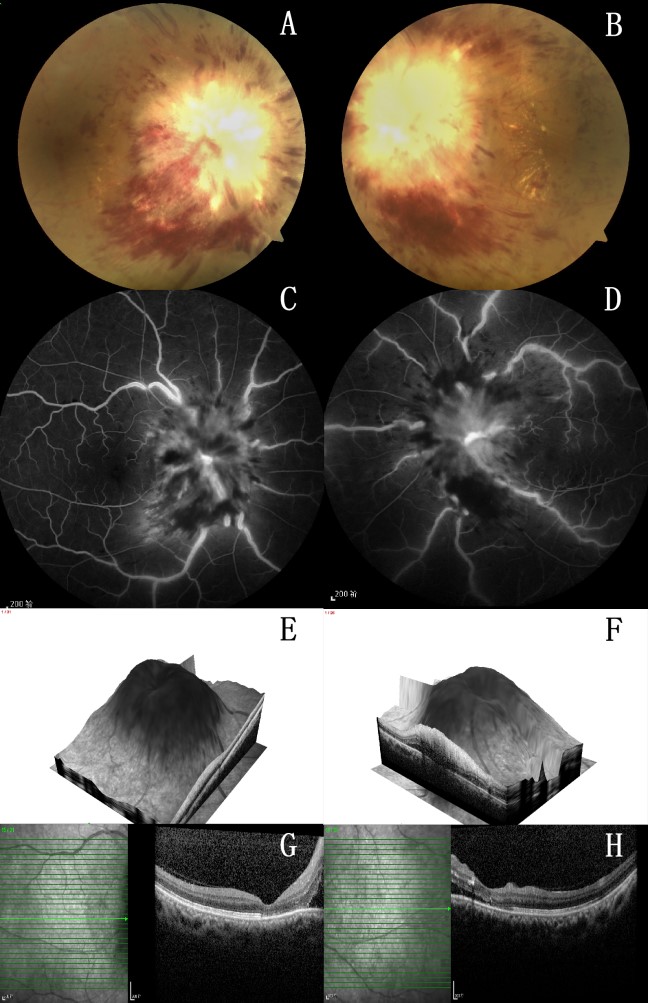
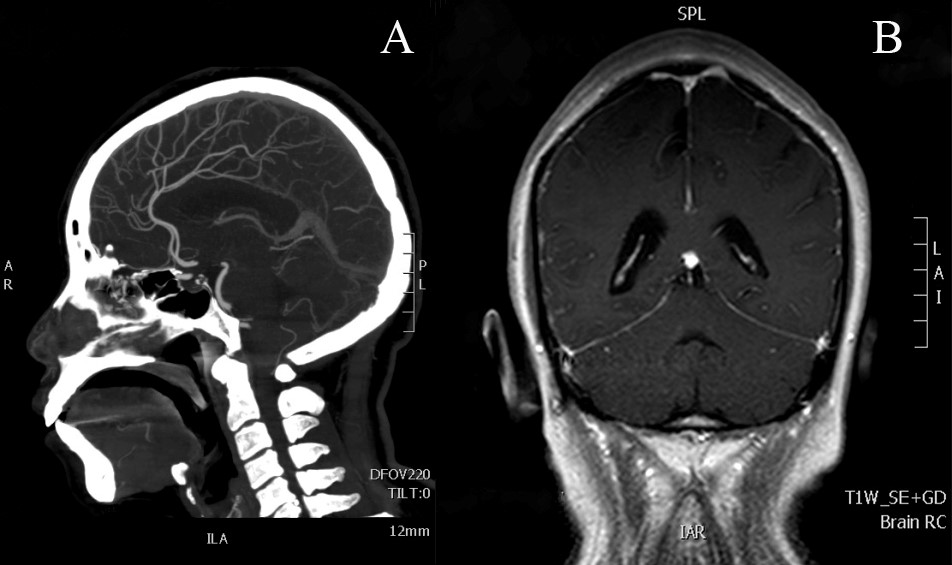
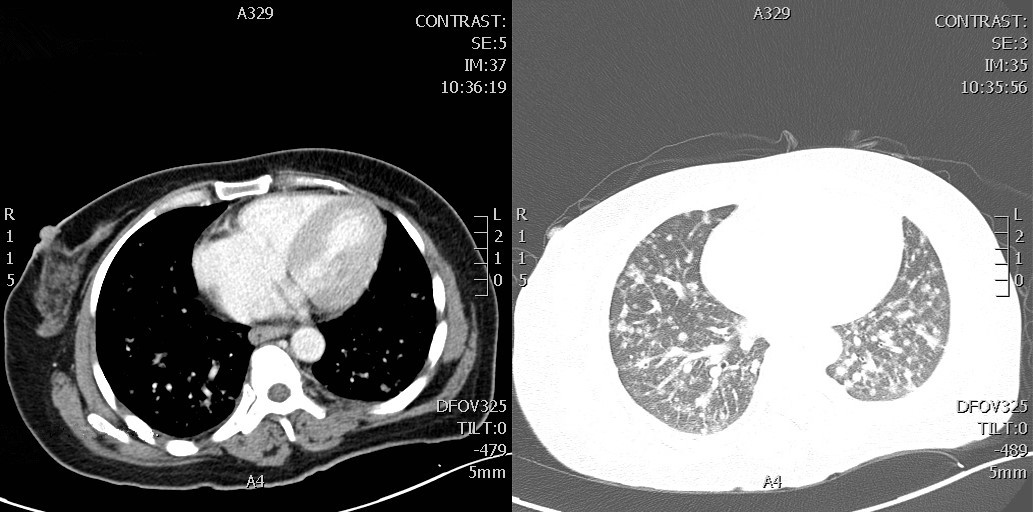
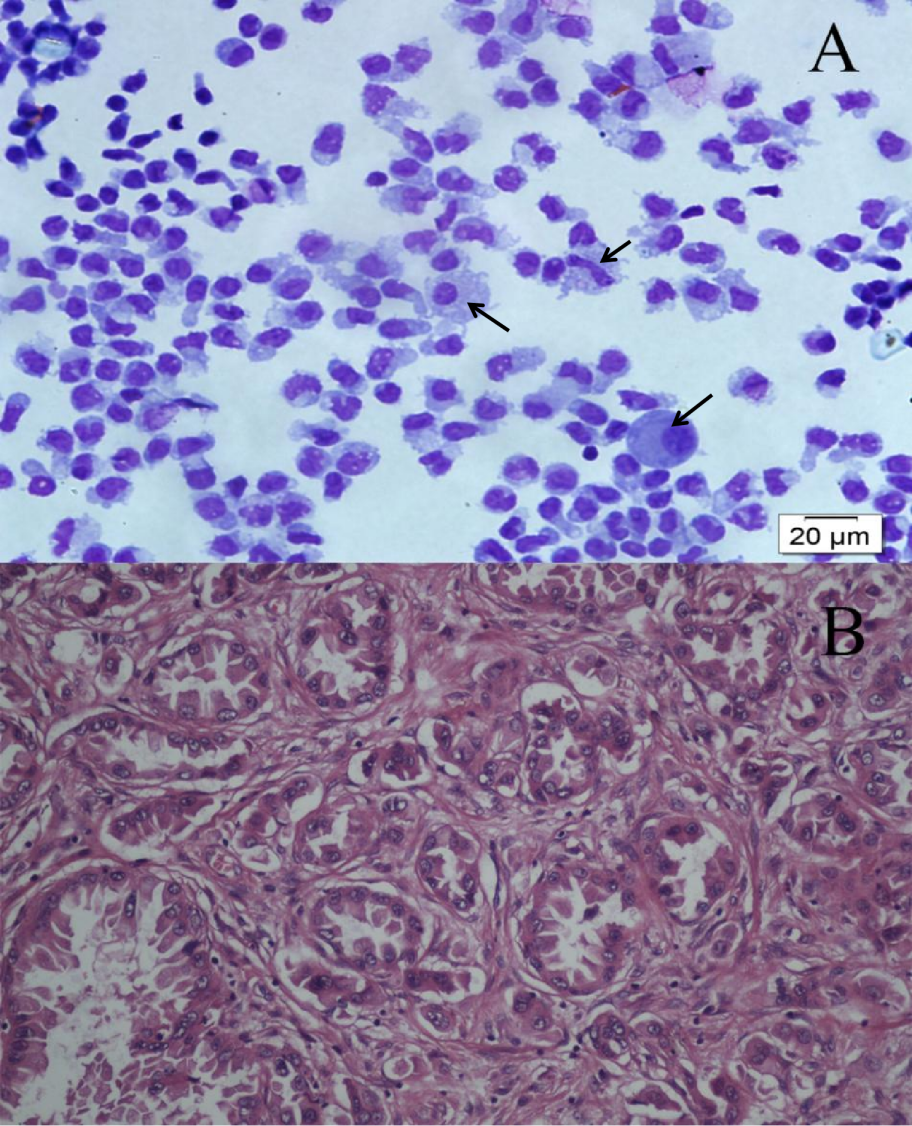
1、Kaplan JG, DeSouza TG, Farkash A, et al. Leptomeningeal
metastases: comparison of clinical features and laboratory
data of solid tumors, lymphomas and leukemias. J Neuro
Oncol. 1990, 9(3): 225-229. DOI: 10.1007/BF02341153.Kaplan JG, DeSouza TG, Farkash A, et al. Leptomeningeal
metastases: comparison of clinical features and laboratory
data of solid tumors, lymphomas and leukemias. J Neuro
Oncol. 1990, 9(3): 225-229. DOI: 10.1007/BF02341153.
2、Kesari S, Batchelor TT. Leptomeningeal metastases.
Neurol Clin. 2003, 21(1): 25-66. DOI: 10.1016/s0733-
8619(02)00032-4.Kesari S, Batchelor TT. Leptomeningeal metastases.
Neurol Clin. 2003, 21(1): 25-66. DOI: 10.1016/s0733-
8619(02)00032-4.
3、Nayar G, Ejikeme T, Chongsathidkiet P, et al.
Leptomeningeal disease: current diagnostic and therapeutic
strategies. Oncotarget. 2017, 8(42): 73312-73328. DOI:
10.18632/oncotarget.20272.Nayar G, Ejikeme T, Chongsathidkiet P, et al.
Leptomeningeal disease: current diagnostic and therapeutic
strategies. Oncotarget. 2017, 8(42): 73312-73328. DOI:
10.18632/oncotarget.20272.
4、Thakkar JP, Kumthekar P, Dixit KS, et al. Leptomeningeal
metastasis from solid tumors. J Neurol Sci. 2020,411 :
116706. DOI:10.1016/j.jns.2020.116706.Thakkar JP, Kumthekar P, Dixit KS, et al. Leptomeningeal
metastasis from solid tumors. J Neurol Sci. 2020,411 :
116706. DOI:10.1016/j.jns.2020.116706.
5、El Rassy E, Botticella A, Kattan J, et al. Non-small cell
lung cancer brain metastases and the immune system: from
brain metastases development to treatment. Cancer Treat
Rev. 2018, 68: 69-79. DOI: 10.1016/j.ctrv.2018.05.015.El Rassy E, Botticella A, Kattan J, et al. Non-small cell
lung cancer brain metastases and the immune system: from
brain metastases development to treatment. Cancer Treat
Rev. 2018, 68: 69-79. DOI: 10.1016/j.ctrv.2018.05.015.
6、Nayak L, Lee EQ, Wen PY. Epidemiology of brain
metastases. Curr Oncol Rep. 2012, 14(1): 48-54. DOI:
10.1007/s11912-011-0203-y.Nayak L, Lee EQ, Wen PY. Epidemiology of brain
metastases. Curr Oncol Rep. 2012, 14(1): 48-54. DOI:
10.1007/s11912-011-0203-y.
7、Chamberlain MC, Glantz M, Groves MD, et al. Diagnostic
tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of
treatment. Semin Oncol. 2009,36(4 Suppl 2): S35-S45.
DOI:10.1053/j.seminoncol.2009.05.005Chamberlain MC, Glantz M, Groves MD, et al. Diagnostic
tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of
treatment. Semin Oncol. 2009,36(4 Suppl 2): S35-S45.
DOI:10.1053/j.seminoncol.2009.05.005
8、Wa l z J . O c u l a r m a n i f e s t a t i o n s o f m e n i n g e a l
carcinomatosis: a case report and literature review.
Optometry. 2011, 82(7): 408-412. DOI: 10.1016/
j.optm.2010.12.015.Wa l z J . O c u l a r m a n i f e s t a t i o n s o f m e n i n g e a l
carcinomatosis: a case report and literature review.
Optometry. 2011, 82(7): 408-412. DOI: 10.1016/
j.optm.2010.12.015.
9、Balm M, Hammack J. Leptomeningeal carcinomatosis.
Presenting features and prognostic factors. Arch
Neurol. 1996, 53(7): 626-632. DOI: 10.1001/
archneur.1996.00550070064013.Balm M, Hammack J. Leptomeningeal carcinomatosis.
Presenting features and prognostic factors. Arch
Neurol. 1996, 53(7): 626-632. DOI: 10.1001/
archneur.1996.00550070064013.
10、Li YS, Jiang BY, Yang JJ, et al. Unique genetic profiles
from cerebrospinal fluid cell-free DNA in leptomeningeal
metastases of EGFR-mutant non-small-cell lung cancer:
a new medium of liquid biopsy. Ann Oncol..2018, 29(4):
945-952. DOI:10.1093/annonc/mdy009.Li YS, Jiang BY, Yang JJ, et al. Unique genetic profiles
from cerebrospinal fluid cell-free DNA in leptomeningeal
metastases of EGFR-mutant non-small-cell lung cancer:
a new medium of liquid biopsy. Ann Oncol..2018, 29(4):
945-952. DOI:10.1093/annonc/mdy009.
11、Zhao Y, He JY, Zou YL, et al. Evaluating the cerebrospinal
fluid ctDNA detection by next-generation sequencing in
the diagnosis of meningeal Carcinomatosis. BMC Neurol.
2019, 19(1): 331. DOI: 10.1186/s12883-019-1554-5.Zhao Y, He JY, Zou YL, et al. Evaluating the cerebrospinal
fluid ctDNA detection by next-generation sequencing in
the diagnosis of meningeal Carcinomatosis. BMC Neurol.
2019, 19(1): 331. DOI: 10.1186/s12883-019-1554-5.
12、Zhao Y, He JY, Cui JZ, et al. Detection of genes mutations
in cerebrospinal fluid circulating tumor DNA from
neoplastic meningitis patients using next generation
sequencing. BMC Cancer. 2020, 20(1): 690. DOI: 10.1186/
s12885-020-07172-x.Zhao Y, He JY, Cui JZ, et al. Detection of genes mutations
in cerebrospinal fluid circulating tumor DNA from
neoplastic meningitis patients using next generation
sequencing. BMC Cancer. 2020, 20(1): 690. DOI: 10.1186/
s12885-020-07172-x.
13、Chamberlain MC, Sandy AD, Press GA. Leptomeningeal
metastasis: a comparison of gadolinium-enhanced MR and
contrast-enhanced CT of the brain. Neurology. 1990, 40(3
Pt 1): 435-438. DOI: 10.1212/wnl.40.3_part_1.435.Chamberlain MC, Sandy AD, Press GA. Leptomeningeal
metastasis: a comparison of gadolinium-enhanced MR and
contrast-enhanced CT of the brain. Neurology. 1990, 40(3
Pt 1): 435-438. DOI: 10.1212/wnl.40.3_part_1.435.
14、Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous
meningitis: Leptomeningeal metastases in solid tumors.
Surg Neurol Int. 2013, 4(Suppl 4): S265-S288. DOI:
10.4103/2152-7806.111304.Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous
meningitis: Leptomeningeal metastases in solid tumors.
Surg Neurol Int. 2013, 4(Suppl 4): S265-S288. DOI:
10.4103/2152-7806.111304.
15、Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal
carcinomatosis in non–small-cell lung cancer patients:
impact on survival and correlated prognostic factors. J Thorac Oncol. 2013, 8(2): 185-191. DOI: 10.1097/
jto.0b013e3182773f21.Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal
carcinomatosis in non–small-cell lung cancer patients:
impact on survival and correlated prognostic factors. J Thorac Oncol. 2013, 8(2): 185-191. DOI: 10.1097/
jto.0b013e3182773f21.
16、Kokkoris%20CP.%20Leptomeningeal%20carcinomatosis.%20How%20does%20%0Acancer%20reach%20the%20pia-arachnoid%3F%20Cancer.%201983%2C%2051(1)%3A%20154-%0A160.%20DOI%3A%2010.1002%2F1097-0142(19830101)51%3A%201%3C154%3A%20aid%02cncr2820510130%3E3.0.co%3B2-k.Kokkoris%20CP.%20Leptomeningeal%20carcinomatosis.%20How%20does%20%0Acancer%20reach%20the%20pia-arachnoid%3F%20Cancer.%201983%2C%2051(1)%3A%20154-%0A160.%20DOI%3A%2010.1002%2F1097-0142(19830101)51%3A%201%3C154%3A%20aid%02cncr2820510130%3E3.0.co%3B2-k.
17、Scanlon, E F, and S Murthy. The process of metastasis.
CA: a cancer journal for clinicians vol. 41,5 (1991): 301-5.
DOI:10.3322/canjclin.41.5.301.Scanlon, E F, and S Murthy. The process of metastasis.
CA: a cancer journal for clinicians vol. 41,5 (1991): 301-5.
DOI:10.3322/canjclin.41.5.301.
18、Lin MS. Subdural lesions linking additional intracranial
spaces and chronic subdural hematomas: a narrative
review with mutual correlation and possible mechanisms
behind high recurrence. Diagnostics. 2023, 13(2): 235.
DOI: 10.3390/diagnostics13020235.Lin MS. Subdural lesions linking additional intracranial
spaces and chronic subdural hematomas: a narrative
review with mutual correlation and possible mechanisms
behind high recurrence. Diagnostics. 2023, 13(2): 235.
DOI: 10.3390/diagnostics13020235.
19、Wasserstrom, W R et al. Diagnosis and treatment of
leptomeningeal metastases from solid tumors: experience
with 90 patients. Cancer. 1982 49 (4): 759-772.
DOI:10.1002/1097-0142(19820215)49:4<759::aid�cncr2820490427>3.0.co;2-7.Wasserstrom, W R et al. Diagnosis and treatment of
leptomeningeal metastases from solid tumors: experience
with 90 patients. Cancer. 1982 49 (4): 759-772.
DOI:10.1002/1097-0142(19820215)49:4<759::aid�cncr2820490427>3.0.co;2-7.
20、McFadzean R, Brosnahan D, Doyle D, et al. A diagnostic
quartet in leptomeningeal infiltration of the optic nerve
sheath. J Neuroophthalmol. 1994,14(3),: 175-182.
DOI:10.3109/01658109409024045.McFadzean R, Brosnahan D, Doyle D, et al. A diagnostic
quartet in leptomeningeal infiltration of the optic nerve
sheath. J Neuroophthalmol. 1994,14(3),: 175-182.
DOI:10.3109/01658109409024045.
21、Corbin, Zachary A, and Seema Nagpal. Leptomeningeal
Metastases. JAMA. 2016, 2(6): 839.
DOI: 10.1001/jamaoncol.2015.3502.Corbin, Zachary A, and Seema Nagpal. Leptomeningeal
Metastases. JAMA. 2016, 2(6): 839.
DOI: 10.1001/jamaoncol.2015.3502.
22、Gauthier H, Guilhaume MN, Bidard FC, et al. Survival of
breast cancer patients with meningeal carcinomatosis. Ann
Oncol. 2010,21(11) : 2183-2187. DOI:10.1093/annonc/
mdq232.Gauthier H, Guilhaume MN, Bidard FC, et al. Survival of
breast cancer patients with meningeal carcinomatosis. Ann
Oncol. 2010,21(11) : 2183-2187. DOI:10.1093/annonc/
mdq232.
23、Mack%20F%2C%20Baumert%20BG%2C%20Sch%C3%A4fer%20N%2C%20et%20al.%20Therapy%20of%20%0Aleptomeningeal%20metastasis%20in%20solid%20tumors.%20Cancer%20Treat%20%0ARev.%202016%2C%2043%3A%2083-91.%20DOI%3A%2010.1016%2Fj.ctrv.2015.12.004.Mack%20F%2C%20Baumert%20BG%2C%20Sch%C3%A4fer%20N%2C%20et%20al.%20Therapy%20of%20%0Aleptomeningeal%20metastasis%20in%20solid%20tumors.%20Cancer%20Treat%20%0ARev.%202016%2C%2043%3A%2083-91.%20DOI%3A%2010.1016%2Fj.ctrv.2015.12.004.
24、Rudnicka H, Niwińska A, Murawska M. Breast cancer
leptomeningeal metastasis: the role of multimodality
treatment. J Neuro Oncol. 2007, 84(1): 57-62. DOI:
10.1007/s11060-007-9340-4.Rudnicka H, Niwińska A, Murawska M. Breast cancer
leptomeningeal metastasis: the role of multimodality
treatment. J Neuro Oncol. 2007, 84(1): 57-62. DOI:
10.1007/s11060-007-9340-4.