Background: To compare objective electrophysiological contrast sensitivity function (CSF) in patients implanted with either multifocal intraocular lenses (MIOLs) or monofocal intraocular lenses (IOLs) by pattern reversal visual evoked potentials (prVEP) measurements.
Methods: Fourty-five cataract patients were randomly allocated to receive bilaterally: apodized diffractive-refractive Alcon Acrysof MIOL (A), full diffractive AMO Tecnis MIOL (B) or monofocal Alcon Acrysof IOL (C). Primary outcomes: 1-year differences in objective binocular CSF measured by prVEP with sinusoid grating stimuli of 6 decreasing contrast levels at 6 spatial frequencies. Secondary outcomes: psychophysical CSF measured with VCTS-6500, photopic uncorrected distance (UDVA), and mesopic and photopic uncorrected near and intermediate visual acuities (UNVA and UIVA respectively).
Results: Electrophysiological CSF curve had an inverted U-shaped morphology in all groups, with a biphasic pattern in Group B. Group A showed a lower CSF than group B at 4 and 8 cpd, and a lower value than group C at 8 cpd. Psychophysical CSF in group A exhibited a lower value at 12 cpd than group B. Mean photopic and mesopic UNVA and UIVA were worse in monofocal group compared to the multifocal groups. Mesopic UNVA and UIVA were better in group B.
Conclusions: Electrophysiological CSF behaves differently depending on the types of multifocal or monofocal IOLs. This may be related to the visual acuity under certain conditions or to IOL characteristics. This objective method might be a potential new tool to investigate on MIOL differences and on subjective device-related quality of vision.
Activities of daily living, such as driving an automobile, confront the individual with an ever-changing set of visual targets, luminances, and contrasts that require rapid visual interpretation. The achievement of this goal could be challenging especially in presbyopic or pseudophakic patient, due to the loss of accommodation. This has led to the development of multifocal intraocular lenses (MIOLs) and pseudo accommodative intraocular lenses (IOLs). Generally, MIOLs with full diffractive or apodized diffractive-refractive optics provide better near visual acuity when compared with accommodative or purely refractive MIOLs (1-4).
Though diffractive MIOLs are well suited for cataract patients who want to be free of spectacles, they still present side effects such as glare and halos. Further, some MIOLs remain inconsistent in achieving adequate distance and near vision (1-7).
The associated photic phenomena are mainly due to difficulties to attend the in-focus image and disregard the superimposed out-of-focus image(s) produced by MIOLs, which is related to the reduced contrast sensitivity (CS) (8,9).
We know that CS is a critical parameter in the assessment of visual performance and ability to function in real life in the evaluation of new medical devices and patient’s satisfaction (10). Many clinical studies have underlined as MIOLs, due to the distribution of light energy between two or more focal points, have a negative impact on psychophysical contrast sensitivity with respect to monofocal IOLs. The loss of CS is less serious in binocular vision, especially with newer generation MIOLs which, due to diffractive aspheric design, approached in some reports the level of monofocal IOLs in terms of mesopic and photopic CS, with or without glare (2,4,11-16).
Some studies showed sine-wave grating CS curve reduction, especially at higher spatial frequencies and in mesopic conditions, with MIOLs compared with monofocal or accommodative IOLs, whereas others found a reduction at all or only at lower frequencies (4,5,13,17,18).
Differences in CS between apodized and full diffractive MIOLs were denied in some studies and described in others, with a better mesopic contrast sensitivity at low to mid spatial frequencies with hybrid apodized ReSTOR +3.0 D compared with full diffractive Tecnis ZMA00 +4.0 D MIOL in one study (8,13,19). Due to these variable and sometimes conflicting results in terms of psychophysical contrast sensitivity function (CSF) in MIOLs, we analyzed literature data regarding the objective measurement of CSF using visual evoked potentials (VEPs). Many longtime studies, based on the “regression technique” for determining contrast thresholds, have demonstrated that when VEPs are recorded for a series of stimulus contrasts and VEP amplitude is extrapolated to zero, the corresponding threshold closely matches the subjective threshold, with VEP prediction accuracy of psychophysical threshold generally satisfactory in normal subject (19-24).
Very few reports exist of electrophysiological CS evaluation using the same tool in pseudophakic subjects, factors such as time consumption, reduced sensitivity, and irregularities in the VEPs signals, caused the test being regarded as impractical for clinical use (21,23,25).
Notwithstanding, taking into account the reliability and time sparing allowed by modern visual electrodiagnostic systems, we decided to evaluate the VEP-based CSF in cataractous patients bilaterally implanted with 2 different diffractive MIOLs, and to compare the results to those obtained under the same conditions with monofocal IOLs.
To the best of our knowledge, this is the first attempt to analyze objective electrophysiological CSF with a modern apparatus in MIOLs-implanted subjects.
After approval by the Ethics Committee of the “Paolo Giaccone” University Hospital in Palermo (ID 09/2011), 45 patients (90 eyes) with bilateral cataracts were enrolled consecutively in a randomized, prospective clinical trial to receive bilaterally one of the three IOL types: the apodized diffractive and refractive Alcon Acrysof IQ ReSTOR SN6AD1 +3.00 D add MIOL (Alcon Laboratories, Inc, Irvine, CA, USA), the full diffractive AMO Tecnis ZMA00 +4.00 D MIOL (Abbott Medical Optics, Santa Ana, CA, USA) or the Alcon Acrysof SN60WF monofocal IOL (Alcon Laboratories).
Inclusion criteria included bilateral juvenile or senile cataract, visually significant [i.e., corrected distance visual acuity (CDVA) >0.2 logMAR] in at least 1 eye.Randomization used a 1:1:1 block randomization scheme generated by the Statistical Package for the Social Sciences (Windows software version 22.0, IBM Corporation, Armonk, NY, USA).
The potential benefits and drawbacks of monofocal and multifocal IOLs were explained, including optimal far acuity and CS, but spectacle dependence for near with the monofocals, and reduced spectacle dependence, better uncorrected near visual acuity (UNVA), and possible glare and halos with MIOLs. Written informed consent were conducted in accord with the tenets of the declaration of Helsinki.
Exclusion criteria included more than 1.00 D of corneal astigmatism, age <18 years; pre-cataract myopia or hyperopia >3 D, history of amblyopia, fundus abnormalities that could cause significant vision impairment, previous surgical intraocular procedures; and ocular comorbidities, such as previous trauma, glaucoma, diabetic retinopathy, pseudoexfoliation syndrome, chronic uveitis, corneal opacities, and alpha-antagonist (e.g., tamsulosin) treatment, intraoperative complications such as iris pupillary trauma, vitreous loss, and inability to place the IOL in the capsular bag.
Infrared computerized pupillometry, keratometry by topographic examination (Sirius CSO, Florence, Italy), and immersion ultrasound biometry (OcuScanRxP, Alcon Laboratories, Inc, Ft. Worth, TX, USA) were performed in all cases by two experienced examiners (Giovanni Cillino or Viviana Firpo). Emmetropia was targeted and IOL power was determined with the Sanders-Retzlaff-Kraft Theoretical formula in both eyes of all patients.
The medical staff who collected functional data (V Firpo, G Maniscalco, S Di Naro, A Iggui) were masked to the type of lens that each patient received. The randomization code was maintained only at the central data facility and was not broken until all data analysis was complete.
The 6-mm acrylic optical surface of the aspheric hybrid diffractive/refractive Alcon ReSTOR SN6AD1 IOL is refractive at the periphery for distance vision and apodized diffractive at the central 3.6 mm of the anterior surface for distance and near vision. The IOLs have 0° angled optics and should add ?0.20 μm of spherical aberration to the eye at the 6-mm optical zone. Apodization means that the diffractive steps are greater in the center of the IOL to give a greater proportion of light to near vision with miotic pupils and to favor distance vision when pupils enlarge. The apodized diffractive 3.6-mm central area of the +3.00 D IOL consists of 9 concentric steps of gradually decreasing height, creating bifocality from near to far and providing +2.40 D near add at the lens plane, with better intermediate vision or extended reading distance (26-28).
The AMO Tecnis ZMA00 with +4.00 D add has a 6-mm acrylic full diffractive optic. The posterior surface of the IOL contains a diffractive multifocal pattern, with a central 1-mm refractive area, and the anterior surface is a modified prolate refractive zone. The anterior surface is wavefront designed and intended to reduce the total amount of aberration and improve mesopic CS by introducing negative spherical aberration into the eye’s optical system. The IOL has a 5° angled optic design and should introduce ?0.27 mm of spherical aberration to the eye measured at the 6-mm optical zone. The diffractive pattern is 32 concentric circles with a +4.00 D near add that creates an even split of the light distribution between near and distance vision, regardless of pupil diameter, with approximately +3.00 D at the lens plane (26,29). The Alcon Acrysof SN60WF monofocal IOL is a single-piece hydrophobic acrylic IOL and it has a yellow-tinted surface with a negative spherical aberration to compensate for the positive aberration of an average cornea; it is engineered to excel in low-light conditions, improving contrast sensitivity and functional vision (30,31).
Surgeries were performed by 1 of 2 experienced surgeons (Salvatore Cillino or Giovanni Cillino). All patients underwent sutureless cataract surgery technique through a temporal 2.6-mm near-clear corneal tunnel incision with a precalibrated knife (Clearcut, Alcon Italia S.P.A., Milan, Italy). Phacoemulsification was performed with the Alcon Infiniti Vision System (Alcon Italia S.P.A.). The Tecnis ZMA00 IOL was inserted using an Unfolder Emerald injector system, the ReSTOR SN6AD IOLs and the Acrysof SN60WF monofocal IOLs were implanted using an Alcon Monarch II injector. The surgical wound was closed by stromal hydration. Surgery in the second eye was performed 1 month later, with the same type of IOL implanted in the second eye. All patients received topical ofloxacin (Exocin, Allergan SpA, Rome, Italy) for 3 days preoperatively and tobramycin and dexamethasone ophthalmic suspension (Tobradex, Alcon Italia S.P.A., Milan, Italy) for 4 weeks postoperatively.
The 45 patients were examined at the Ophthalmology Department of Palermo University Hospital at day 1, week 1, months 1, 3 and 12. Ophthalmic examination included biomicroscopy, manifest refraction, intraocular pressure measurement, fundoscopy and evaluation of postoperative posterior capsular opacity.
Primary outcome, evaluated at 12 months was the objective binocular CSF function measured by pattern reversal Visual Evoked Potentials (prVEP) among the 3 IOL implanted groups.
Secondarily, we recorded the photopic uncorrected distance visual acuity (UDVA), the mesopic and photopic uncorrected near and intermediate visual acuity (UNVA and UIVA respectively), and the photopic psychophysical CSF, measured with Vision Contrast Test System VCTS-6500.
A UTAS Visual Electrodiagnostic Testing System apparatus (LKC Technologies, Gaithersburg, MD, USA), measuring the electrical response of the primary visual cortex when visually stimulated, was employed (32).
The stimulus pattern was displayed on a 17-inch, high resolution 75 Hz DVI LCD monitor (Lenovo Mod. LT1713p, Segrate, Milan, Italy). The overall stimulus field was 22.5×22.5 cm. The stimulus field size subtended a visual angle of 16×16° at the testing distance of 80 cm. The mean luminance of the monitor was maintained at a low-photopic level of 40 candelas (cd)/m2, under dim ambient light conditions, in order to avoid extreme miosis and to obtain a near mesopic pupillary diameter, to eventually emphasize the differences in light focusing among IOLs.
The stimuli were black and white vertically oriented (90°) sinewave gratings, which changed phase at a temporal frequency of 8 Hz (pattern reversal), without overall change in the luminance of the screen, with equal numbers of light and dark elements in the display, and no transient luminance change during pattern reversal. International Society for Clinical Electrophysiology of Vision (ISCEV) 2009 Standard was used to set all parameters (33).
Steady state prVEP were obtained while the patients watching the sinusoidal grating stimuli with undilated pupils. Six progressively decreasing contrast levels (100%, 64%, 52%, 24%, 16%, 8%) were swept at six spatial frequencies: 0.25, 0.5, 1.0, 2.0, 4.0 and 8.0 cycles per degree (cpd). The patients were given short periods of rest between each contrast measurement so as to minimise fatigue and any concomitant increase in response variability. The prVEP protocol to obtain a waveform included 80 responses, which were amplified, filtered via the processing system (0.5–100 Hz), summed and averaged, then memorized, within 11 seconds. Therefore, to analyze the whole range of frequencies at one contrast level required little more than one minute, while the whole examination lasted about 10 minutes. The P100 wave amplitude in μV was calculated at each spatial frequency for each contrast level.
Contrast sensitivity data for each group of patients were best fit with a linear function when the data were plotted in log–linear coordinates, i.e., log contrast sensitivity as a function of spatial frequency. In particular, the contrast threshold was determined as follows: after the recordings, the best-fitting straight line through the six contrast-related prVEPs responses in μV at each spatial frequency was calculated. The contrast threshold for each frequency was estimated by plotting the amplitude of these responses against log-contrast and extrapolating the linear model to a 0-μV amplitude level, using the Statistical Package for the Social Sciences (SPSS Software 22.0 version, IBM Corp., Armonk, NY, USA) (Figure 1).
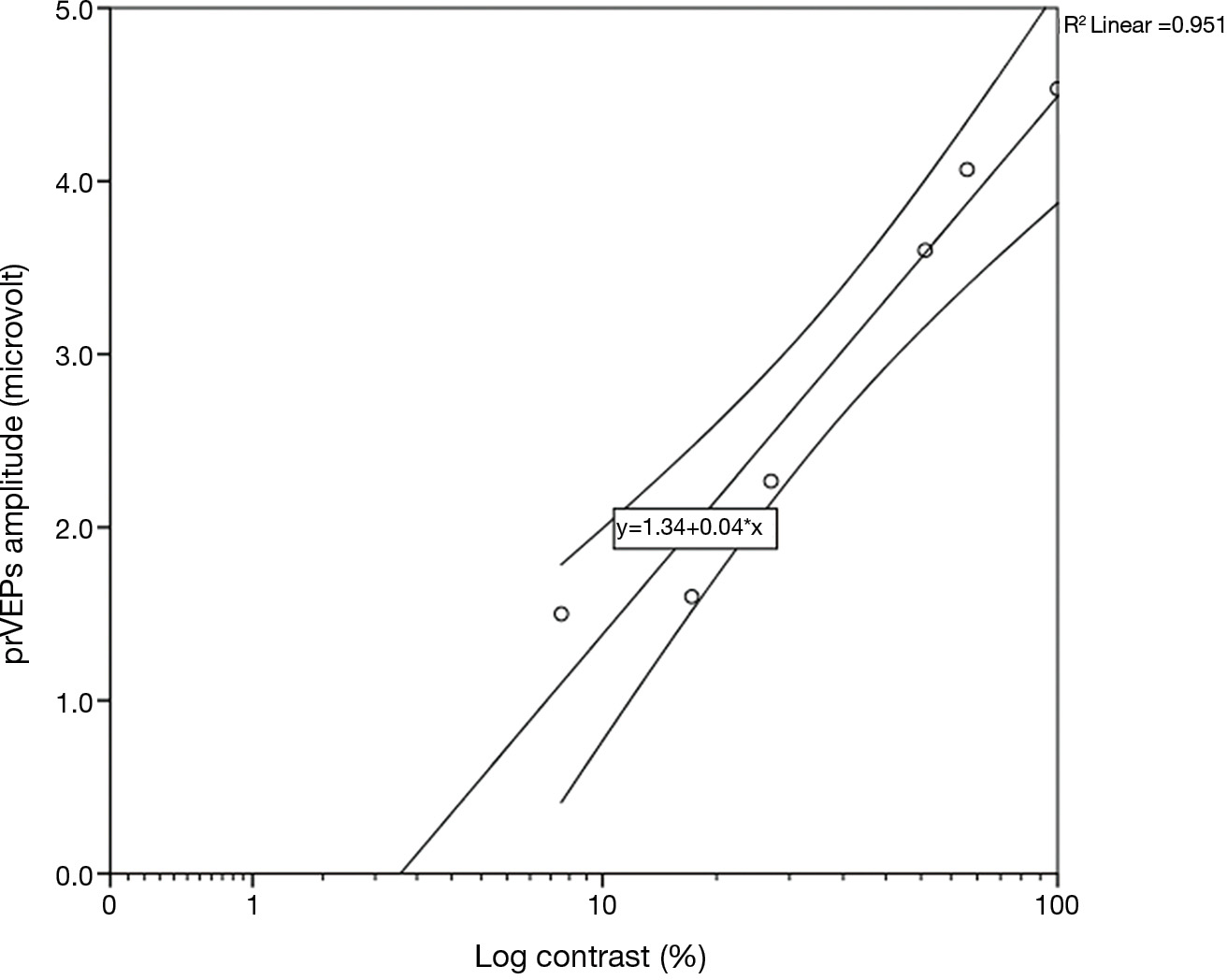
Hence, the reciprocal values of six contrast thresholds, one for each spatial frequency, derived from amplitude measurements, were plotted against all spatial frequencies, obtaining the log CSF curve in each group, since log contrast sensitivity = log 1/contrast threshold. The logarithm of contrast sensitivity is generally preferred due to its normal distribution, suitable for further statistical comparisons (24).
Psychophysical contrast sensitivity was determined binocularly by Vision Contrast Test System, VCTS-6500 (Vistech Consultants, Inc, Dayton, OH, USA), under photopic conditions at 85 cd/m2. These charts consist of sine wave gratings. Each chart contains five rows and nine columns of circular photographic plates (disc) on gray background. Each row has different spatial frequency (1.5, 3, 6, 12 and 18 cpd at 3 meters) and contrast within the row reduces from left to right. The gratings are presented in three orientations: vertical 90 degrees, 15 degrees clockwise or anti-clockwise. The lowest contrast grating determines the sensitivity score for that spatial frequency (34). Scores arising from VCTS-6500 were plotted using a Log CS scale, instead of the contrast sensitivity percentage reported in the evaluation form, to be consistent with the electrophysiological CSF.
Preoperative CDVA and postoperative binocular UDVA were measured in logMAR notation at 100% contrast using Early Treatment of Diabetic Retinopathy Study charts under photopic conditions (CC-100XP LCD System for Chart display, Topcon Europe BV, Milano, Italy) at 3 m. Postoperative binocular UNVA and UIVA were measured using the Federal Aviation Administration Near Vision Acuity Chart (Snellen units converted to logMAR by the Visual Acuity Conversion Chart), with 100% contrast at a mean distance of 35 and 80 cm respectively. These acuities were measured in both photopic (85 cd/m2) and low mesopic (3 cd/m2) luminance (Luxmeter HD 2302.0, Delta OHM, Tecnopound, Ravenna, Italy).
A power calculation showed that a sample size of 14 patients in each group would have 80% power to detect a difference of 0.15 in log contrast sensitivity values with a standard deviation of 0.2 and significance of 0.05 (two tailed). All continuous data are expressed as a mean ± standard deviation of the mean. Statistical analysis of quantitative data, included descriptive statistics, was performed for all the items. Categorical variables were compared using the Pearson’s chi-square test. For parametric analysis univariate analysis of variance (ANOVA test) with Bonferroni post hoc comparison was used to compare results among the ?3 IOL groups. Data were analyzed by the Epi Info software (version 6.0, Centers for Disease Control and Prevention, Atlanta, GA, USA) and by IBM SPSS Software version 22.0 (IBM Corporation, Armonk, NY, USA). All P values were two sided, and P values less than 0.05 were considered statistically significant.
Between January 2012 and January 2013, 50 cataract patients (100 eyes) were enrolled. Five patients were unable to attend the follow-up schedule, therefore 45 cases (90 eyes) were included in the study. Group A included 16 patients (32 eyes) implanted with the pupil-dependent Alcon ReSTOR SN6AD1 +3.00 D IOL, group B included 14 patients (28 eyes) implanted with the pupil-independent Tecnis ZMA00 +4.00 D IOL and group C included 15 patients (30 eyes) implanted with the Acrysof SN60WF Monofocal IOL. There were no significant preoperative intergroup differences in age, sex, preoperative sphere, and cylinder. Preoperative photopic and mesopic pupil diameters and CDVA were comparable among groups (Table 1). One year after surgery, the three groups did not differ in term of mean manifest refraction, with a residual postoperative spherical power ≤ +0.10 diopters and cylinder power ≤ ?0.25 diopters (Table 2). Far, intermediate and near uncorrected visual acuity values were not significantly different from the 1st month follow-up ones. There was no clinically significant IOL decentration (i.e., >0.3 mm), the posterior capsule maintained adequate transparency for optimal posterior pole biomicroscopy and no macular or optic nerve alterations were found.
| Variable | Group A; ReSTOR SN6AD1; MIOL | Group B; Tecnis; ZMA00; MIOL | Group C; Acrysof SN60WF; IOL | P value |
|---|---|---|---|---|
| Patients, n | 16 | 14 | 15 | ? |
| Eyes, n | 32 | 28 | 30 | ? |
| Gender (male/female) | 9/7 | 7/7 | 7/8 | 0.863* |
| Age (years) ? | 59.9 (12.5) | 64.6 (9.2) | 63.8 (8.7) | 0.449? |
| Sphere (D) ? | 0.55 (2.2) | 0.60 (1.9) | 0.65 (2.0) | 0.930? |
| Cylinder (D) ? | ?0.50 (0.60) | ?0.68 (0.45) | ?0.58 (0.55) | 0.561? |
| CDVA (logMAR) ? | 0.35 (0.24) | 0.28 (0.22) | 0.30 (0.27) | 0.611? |
| Pupil diameter ? photopic | 2.8 (0.5) | 2.8 (0.6) | 2.9 (0.3) | 0.693? |
| Mesopic | 4.0 (0.5) | 4.1 (0.5) | 4.0 (0.3) | 0.702? |
*, Chi square test; ?, mean (±SD); ?, univariate analysis of variance test; CDVA, corrected distance visual acuity; D, diopter; IOL, intraocular lens; logMAR, logarithm of the minimum angle of resolution.
| Variable | Group A (ReSTOR SN6AD1 MIOL) | Group B (Tecnis ZMA00 MIOL) | Group C (Acrysof SN60WF IOL) | P value* | P value (post hoc comparison?) | ||
|---|---|---|---|---|---|---|---|
| A-B | B-C | A-C | |||||
| Pupil diameter (mm) | |||||||
| Photopic | 3.0 (0.4) | 2.8 (0.6) | 2.8 (0.5) | 0.504 | – | – | – |
| Mesopic | 3.9 (0.4) | 4.2 (0.5) | 4.0 (0.3) | 0.239 | – | – | – |
| Sphere (D) ? | 0.07 (0.3) | 0.10 (0.4) | 0.09 (0.5) | 0.887 | – | – | – |
| Cylinder (D) ? | ?0.20 (0.32) | ?0.25 (0.40) | ?0.24 (0.42) | 0.807 | – | – | – |
| UDVA (logMAR) ? | 0.008 (0.05) | 0.006 (0.08) | 0.009 (0.079) | 0.939 | – | – | – |
| UNVA (logMAR) ? | |||||||
| Photopic | 0.02 (0.04) | 0.04 (0.06) | 0.45 (0.07) | 0.001 | 0.286 | <0.0005 | <0.0005 |
| Mesopic | 0.25 (0.07) | 0.18 (0.05) | 0.60 (0.10) | 0.001 | 0.0043 | <0.0005 | <0.0005 |
| UIVA (logMAR) ? | |||||||
| Photopic | 0.07 (0.12) | 0.15 (0.13) | 0.40 (0.08) | 0.001 | 0.090 | <0.0005 | <0.0005 |
| Mesopic | 0.31 (0.07) | 0.23 (0.09) | 0.55 (0.12) | 0.001 | 0.011 | <0.0005 | <0.0005 |
*, univariate analysis of variance test; ?, Bonferroni post hoc comparison; ?, mean (±SD); UDVA, uncorrected distance visual acuity; UNVA, uncorrected near visual acuity; UIVA, uncorrected intermediate visual acuity; D, diopter; MIOL, multifocal intraocular lense; IOL, intraocular lens; logMAR, logarithm of the minimum angle of resolution.
No differences were found in terms of mean pupillary diameters and spherocylinder correction in the 3 groups. All patients achieved a binocular UDVA of <0.010 (i.e., >20/25 Snellen ratio), without significant difference (P=0.939). Mean photopic and mesopic UNVA and UIVA were worse in group C with respect to the two MIOL groups, with values ≥0.40 logMAR (P=0.001). Photopic UNVA did not differ in the two MIOLs groups, while mesopic UNVA was better in group B (P=0.0043). Photopic UIVA did not differ in MIOLs groups, even if a trend favoring group A could be noted (P=0.09), while mesopic UIVA showed better results in group B (P=0.011).
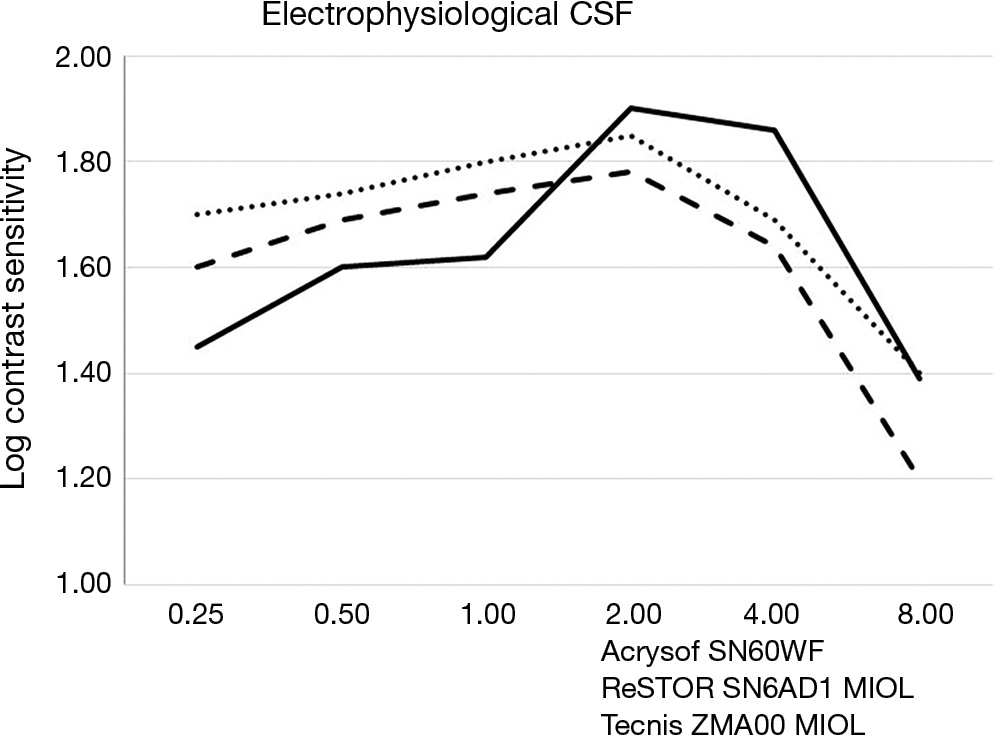
Table 3 shows the mean binocular electrophysiological CSF values. A peak of log CS was observed at 2.00 cpd in all groups, ranging from 1.78 to 1.90, without significant difference. Group B exhibited a lower CS compared to group C at the lowest spatial frequency of 0.25 cpd (P=0.004). From 0.5 to 2.0 cpd the three groups did not differ, while at 4.0 and 8.0 cpd group A showed a lower CS than group B (P=0.031 and 0.048 respectively), and at 8 cpd the same group exhibited a lower value than C (P=0.038). In general, CSF curves in all groups tended to an inverted U-shaped morphology (Figure 2). In more detail, both group A (ReSTOR MIOL) and group C (Acrysof IOL) curves showed a moderate adherence to the abovesaid morphology. Differently, the CSF curve of group B (Tecnis ZMA00 MIOL) showed a more biphasic morphology, with an ascending slope from 0.25 to 0.50 cpd, followed by a plateau, then a second ascending slope from 1.00 to 2.00 cpd, and descending slopes similar to the other two groups thereafter.
| Cpd | Group A (ReSTOR SN6AD1 MIOL) | Group B (Tecnis ZMA00 MIOL) | Group C (Acrysof SN60WF IOL) | P value* | P value (post hoc comparison?) | ||
|---|---|---|---|---|---|---|---|
| A-B | A-C | B-C | |||||
| 0.25 | 1.60 (0.16) | 1.45 (0.17) | 1.70 (0.17) | 0.01 | 0.064 | 0.205 | 0.004 |
| 0.50 | 1.69 (0.20) | 1.60 (0.18) | 1.74 (0.19) | 0.315 | 0.304 | 0.563 | 0.117 |
| 1.00 | 1.74 (0.24) | 1.62 (0.23) | 1.80 (0.24) | 0.324 | 0.278 | 0.583 | 0.111 |
| 2.00 | 1.78 (0.20) | 1.90 (0.21) | 1.85 (0.20) | 0.428 | 0.217 | 0.465 | 0.601 |
| 4.00 | 1.64 (0.25) | 1.86 (0.25) | 1.69 (0.24) | 0.04 | 0.031 | 0.601 | 0.087 |
| 8.00 | 1.20 (0.19) | 1.39 (0.20) | 1.40 (0.20) | 0.04 | 0.048 | 0.038 | 0.912 |
*, univariate analysis of variance test; ?, Bonferroni post hoc comparison; Cpd, cycles per degree; Log CS, mean (±SD) log contrast sensitivity (1/contrast threshold); MIOL, multifocal intraocular lense; IOL, intraocular lense.
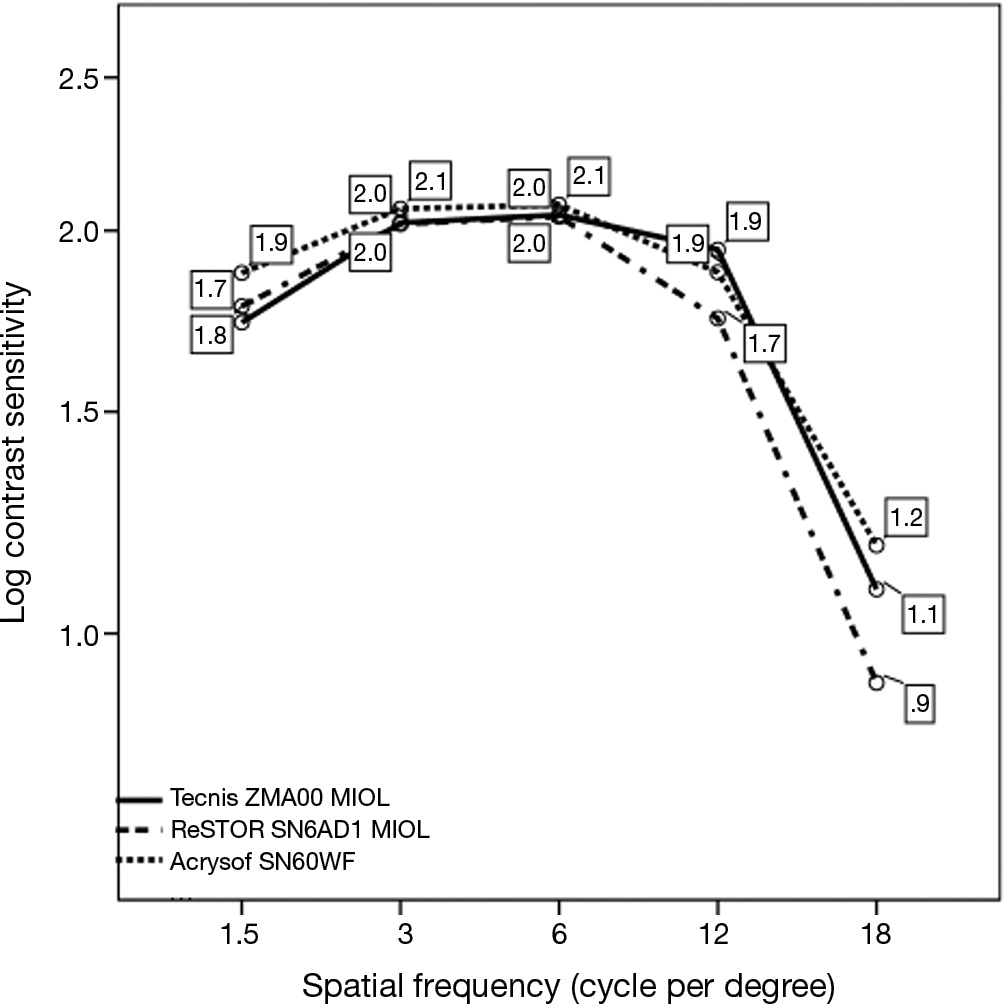
The three groups did not generally differ in terms of binocular photopic psychophysical CSF, peaking at 3 to 6 cpd, with a 2.05 to 2.08 range in Log CS units (Figure 3), equivalent to a 110.25 to 120 contrast sensitivity Vistech score range, except at 1.5 cpd, where group B (Tecnis ZMA00 MIOL) exhibited a lower value than monofocal group C, i.e., 1.74±0.24 vs. 1.88±0.26 log CS units respectively (P=0.038), and at 12 cpd, where group A exhibited a lower value than group B, i.e., 1.74±0.32 vs. 1.94±0.40 log CS units respectively (P=0.0035). In all groups, the curves exhibited the typical inverted U-shaped morphology.
The CSF is a basic measurement of human spatial vision that provides a clinical evaluation of visual function over a wide range of spatial frequencies. CSF measurement is at least as important as high Snellen acuity because it reflects the subject’s visual ability in his or her low contrast living environment in which there are numerous objects. As we mentioned in the Introduction section, MIOLs are typically flawed by a reduced psychophysical contrast sensitivity, especially in mesopic environment, with sometimes-conflicting results in literature data. Factors such as sample size and mean age of the patients, type of test, type of the MIOL optics (full diffractive, diffractive/refractive, apodized, etc), studies not targeted at CS as an outcome, could contribute to the variability of the subjective CSF analysis.
Therefore, we turned to steady state prVEP, which primarily reflects macular function, and can be regarded as useful test for both objective visual acuity and CSF measurement (24). Pattern reversal VEPs was developed as an objective test to circumvent the subject’s consciousness, for example in case of preverbal infants examination, malingerers and all the other situations in which subjective responses of any kind are difficult to obtain (23). Campbell and Maffei, and many authors have then demonstrated the good correlation existing between steady state VEPs electrophysiological response and psychophysical Snellen acuity and CSF, though prVEPs underestimate both parameters when compared to the psychophysical counterpart (19,20,22,35).
Due to the abovesaid reduced VEPs sensitivity with respect to Snellen visual acuity, the minimal and equivalent postoperative refractive error in our groups would not significantly influence the prVEPs response. Our monofocal IOL cases (group C) exhibited as expected an UNVA and UIVA lower than multifocal ones (group A and B). Full diffractive Tecnis MIOL (group B) showed a global better outcome both relating UNVA and UIVA under mesopic conditions with respect to apodized-diffractive ReSTOR MIOL (group A). These findings are linked to the full diffractive aspherically enhanced (?0.27 vs. ?0.20 μm) surface of the Tecnis MIOL, which maintains the 41%/41% light distribution regardless of pupil diameter, whereas the apodized diffractive/refractive surface of the ReSTOR MIOL groups progressively unbalances light distribution to favor distance vision when pupils enlarge (1). These differences on uncorrected visual acuity seem to be in agreement with the prVEPs response in our cases, since we conducted our tests under low-photopic light subtending a visual angle of only 16×16°, at the testing distance of 80 cm, under dim ambient light conditions. Therefore, our patients watched at sinusoidal gratings at the same intermediate distance of the UIVA measurement, under almost mesopic ambient lighting. These premises can explain the poorer performance of group A (apodized diffractive) at the higher spatial frequencies of 4 to 8 cpd, which are more linked to patients’ Snellen visual acuity. At the same spatial frequencies, group B (full diffractive) performed as the monofocal group C, perhaps indicating that the binocular neuro-enhancement with diffractive aspherical optics reduces the typical contrast loss of MIOLs. Other factors contributing to this finding could be a more blurred vision with monofocal IOLs at intermediate distance and the consistently less sensitivity of the electrophysiological CSF with respect to the psychophysical one (24).
However, at the lowest spatial frequency, i.e., 0.25 cpd, group B showed a lower CS with respect to group C. This finding could be related to the biphasic pattern of group B CSF that we will discuss below. Anyway, low frequency responses are more difficult to understand, when considering the reported limitation of pattern VEPs CSF, where a relatively large stimulus field size should be used in order to record good response at low spatial frequencies (24).
In our pseudophakic subjects, the mean binocular electrophysiological CSF curves exhibited a morphology resembling the inverted U-shaped pattern of psychophysical ones. In agreement with previous studies on younger phakic subjects, the U-pattern of these curves was less pronounced than the typical psychophysical response, with a flatter appearance (23,24).
In this regard, we have to note that our electrophysiological CSF curves included responses at very low spatial frequencies, i.e., 0.25 to 1.00 cpd, which are not present in the usual psychophysical tests, such as the Vistech 6500. Moreover, the sinusoidal grating stimuli in our apparatus is limited to 8 cpd, as in the majority of electrophysiological CSF studies, with lack of the typical descending slope at the highest spatial frequencies, i.e., 12 to 18 cpd. On the other hand, the relatively low intermediate visual acuity in our pseudophakic groups should not allow clear responses to higher spatial frequencies. All these factors could justify a morphology flatter than the typical bell-shaped curve found in psychophysical contrast CSF curves.
As described in the Results section, while the patients of group A, implanted with a multifocal IOL (ReSTOR), exhibited a monophasic CSF curve, quite resembling the curve found in monofocal IOL cases of group C (Acrysof), the curve of multifocal IOL cases in group B (Tecnis ZMA00) showed a somehow more biphasic morphology. A hypothetical explanation could derive from the different optics of the MIOLs groups. In fact, as above reported, group A included cases with a diffractive apodized MIOL favoring distance vision when pupils enlarge. Therefore, a near mesopic pupillary diameter could favor light passing through the peripheral part of the optic, with an almost “monofocal” behavior at intermediate distance. Whereas the full diffractive optic of the Tecnis MIOL in group B, pupil-independent which split light rays into two foci, could enhance a behavior already described in healthy phakic subjects. Strasburger et al. (23) found that pattern-reversal contrast functions show large variability between subjects, often, amplitudes are low at intermediate spatial frequencies and in these functions, this might look like a “notch” in an otherwise inverse U-shaped function. They hypothesize that the VEP can be constructed as stemming from two sources, or cellular populations, representing underlying generators of activity, one peaking at low spatial frequencies (transient) and the other peaking at higher ones (sustained). Each subject has its own mix of contributions from these sources, leading to the observed VEP variability. In our study, the higher quality of vision under mesopic conditions at intermediate distance in group B subjects could have emphasized such an intragroup variability.
Regarding the psychophysical CSF in our cases, only a qualitative comparison with the prVEPs results can be done, since the former was performed at a far distance of 3.00 m under photopic conditions at 85 cd/m2, whilst the latter at intermediate distance of 80 cm under low-photopic level of 40 cd/m2 and dim ambient light conditions. We performed though the psychophysical CS examination to ascertain a normal response in our pseudophakic patients The Vistech psychophysical CSF pattern was typical and quite similar among the three groups, with a 2.08 log CS units peak between 3 and 6 cpd. A previous study using FACT contrast sensitivity, which derives from the Vistech one, in phakic subjects of the same age range, indicates a somewhat lower ?3 cpd peak with 1.97±1.58 log CS score (34). This difference from our results could be hypothetically due to the effect of the aging lens in the latter. The lower score of group B at a low frequency row and that of group A at a higher one agree to what we found with the electrophysiological test.
Relating the lack of biphasic curves and the higher log CS values with respect to prVEPs CSF, besides the effect of different experimental conditions, psychophysical contrast enhancement must be taken into account. As reported, the electrophysiological CSF is consistently less sensitive than the psychophysical approach, and the psychophysical threshold is a perception in which only a few neurons need to be activated to obtain the threshold, whereas a greater number of them need to be activated to produce evident electrical potentials in the occipital cortex during the electrophysiological test.
Eventually, we know that contrast sensitivity is not only influenced by the optics of the eye but also by neural influences from retina and cortex. There is no way to determine if the differences in contrast sensitivity (whether measured electrophysiologically or psychophysically) can be attributed to different optical properties and ramifications of the IOLs under study, or individual patient differences in the neural processing of contrast. This bias could be overcome by larger sample size, which is necessary even to confirm our hypotheses on different electrophysiological behaviors.
Our data suggest that the electrophysiological CSF can behave differently according to the types of aspheric diffractive multifocal or monofocal IOLs, and this seems to be related to differences in visual acuity under certain environmental conditions. Differences in the optical characteristics of the IOL could explain differences in CS level at various spatial frequencies and curves pattern. Psychophysical results in our cases confirm that binocular CSF in multifocal IOLs reaches in general the level of the monofocal ones, at least under photopic conditions.
Clearly, technique refinements together with many more data are required to confirm the value and limits of electrophysiological contrast sensitivity in pseudophakic patients. Once these requirements are met, we think that this objective method could be regarded as a potential new tool to investigate on MIOLs differences, advantages and drawbacks, and, ultimately, on device-related quality of vision, giving the surgeon one more tool in the kit to help the patient make an informed decision.