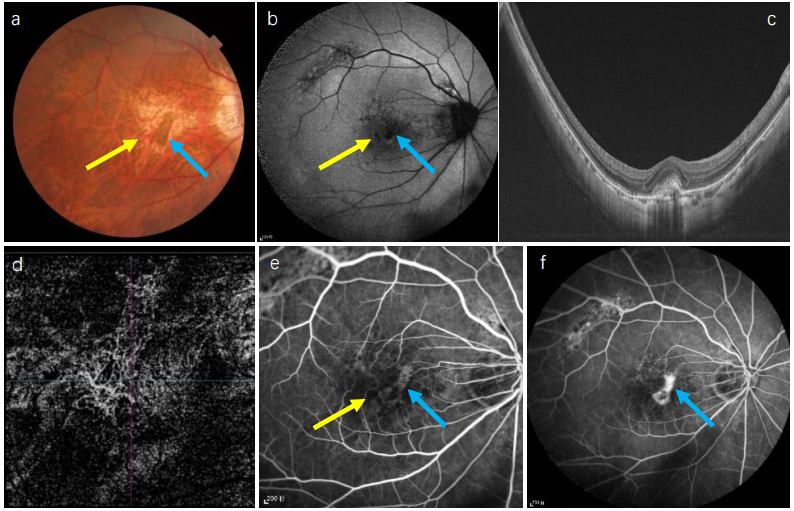
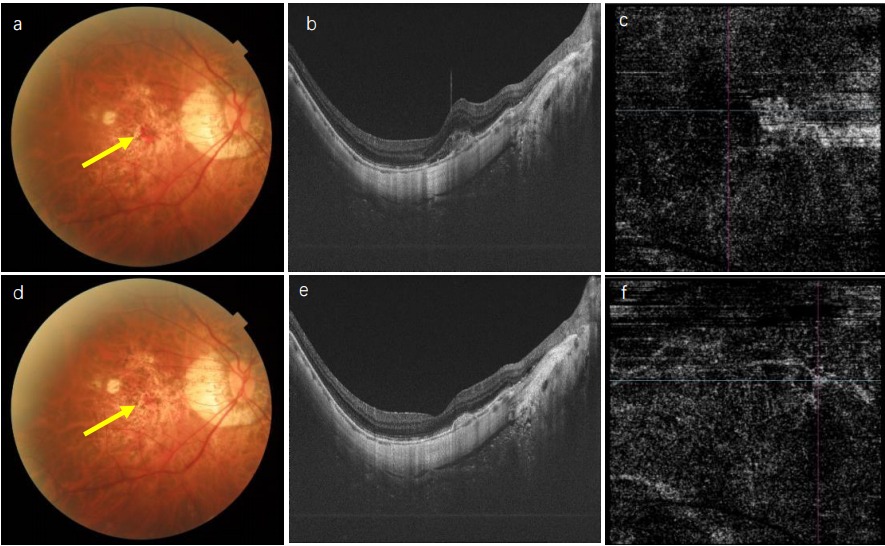
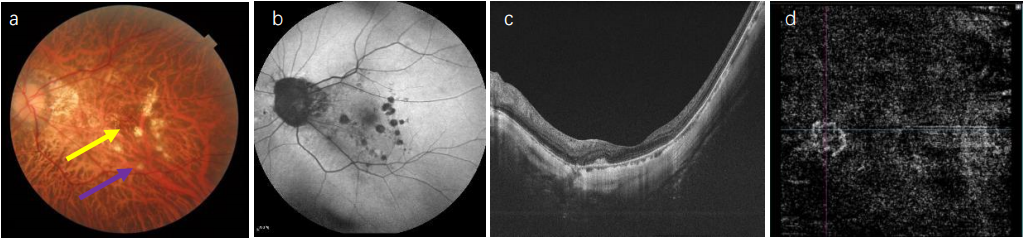
1、Sun J, Zhou J, Zhao P, et al. High prevalence of myopia
and high myopia in 5060 Chinese university students in
Shanghai. Invest Ophthalmol Vis Sci. 2012, 53(12): 7504-
7509. DOI: 10.1167/iovs.11-8343.Sun J, Zhou J, Zhao P, et al. High prevalence of myopia
and high myopia in 5060 Chinese university students in
Shanghai. Invest Ophthalmol Vis Sci. 2012, 53(12): 7504-
7509. DOI: 10.1167/iovs.11-8343.
2、Wong TY, Ferreira A, Hughes R, et al. Epidemiology and
disease burden of pathologic myopia and myopic choroidal
neovascularization: an evidence-based systematic review.
Am J Ophthalmol. 2014, 157(1): 9-25.e12. DOI: 10.1016/
j.ajo.2013.08.010.Wong TY, Ferreira A, Hughes R, et al. Epidemiology and
disease burden of pathologic myopia and myopic choroidal
neovascularization: an evidence-based systematic review.
Am J Ophthalmol. 2014, 157(1): 9-25.e12. DOI: 10.1016/
j.ajo.2013.08.010.
3、Chang L, Pan CW, Ohno-Matsui K, et al. Myopia-related
fundus changes in Singapore adults with high myopia. Am
J Ophthalmol. 2013, 155(6): 991-999.e1. DOI: 10.1016/
j.ajo.2013.01.016.Chang L, Pan CW, Ohno-Matsui K, et al. Myopia-related
fundus changes in Singapore adults with high myopia. Am
J Ophthalmol. 2013, 155(6): 991-999.e1. DOI: 10.1016/
j.ajo.2013.01.016.
4、Haarman AEG, Tedja MS, Brussee C, et al. Prevalence
of myopic macular features in Dutch individuals
of European ancestry with high myopia. JAMA
Ophthalmol. 2022, 140(2): 115-123. DOI: 10.1001/
jamaophthalmol.2021.5346.Haarman AEG, Tedja MS, Brussee C, et al. Prevalence
of myopic macular features in Dutch individuals
of European ancestry with high myopia. JAMA
Ophthalmol. 2022, 140(2): 115-123. DOI: 10.1001/
jamaophthalmol.2021.5346.
5、Quiroz-Mendoza JL, Valera-Cornejo DA, García�Roa M, et al. Different approaches in the management
of macular hemorrhage: case reports and a literature review. Medwave. 2020, 20(2): e7831. DOI: 10.5867/
medwave.2020.02.7831.Quiroz-Mendoza JL, Valera-Cornejo DA, García�Roa M, et al. Different approaches in the management
of macular hemorrhage: case reports and a literature review. Medwave. 2020, 20(2): e7831. DOI: 10.5867/
medwave.2020.02.7831.
6、Ren P, Lu L, Tang X, et al. Clinical features of simple
hemorrhage and myopic choroidal neovascularization
associated with lacquer cracks in pathologic myopia.
Graefes Arch Clin Exp Ophthalmol. 2020, 258(12): 2661-
2669. DOI: 10.1007/s00417-020-04778-6.Ren P, Lu L, Tang X, et al. Clinical features of simple
hemorrhage and myopic choroidal neovascularization
associated with lacquer cracks in pathologic myopia.
Graefes Arch Clin Exp Ophthalmol. 2020, 258(12): 2661-
2669. DOI: 10.1007/s00417-020-04778-6.
7、Chang KJ, Cheng CK, Peng CH. Clinical characteristics
and visual outcome of macular hemorrhage in pathological
myopia with or without choroidal neovascularization.
Taiwan J Ophthalmol. 2016, 6(3): 136-140. DOI: 10.1016/
j.tjo.2016.05.007.Chang KJ, Cheng CK, Peng CH. Clinical characteristics
and visual outcome of macular hemorrhage in pathological
myopia with or without choroidal neovascularization.
Taiwan J Ophthalmol. 2016, 6(3): 136-140. DOI: 10.1016/
j.tjo.2016.05.007.
8、Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy
atrophy and lacquer cracks predispose to the development
of choroidal neovascularisation in pathological myopia.
Br J Ophthalmol. 2003, 87(5): 570-573. DOI: 10.1136/
bjo.87.5.570.Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy
atrophy and lacquer cracks predispose to the development
of choroidal neovascularisation in pathological myopia.
Br J Ophthalmol. 2003, 87(5): 570-573. DOI: 10.1136/
bjo.87.5.570.
9、Wong TY, Ohno-Matsui K, Leveziel N, et al. Myopic
choroidal neovascularisation: current concepts and update
on clinical management. Br J Ophthalmol. 2015, 99(3):
289-296. DOI: 10.1136/bjophthalmol-2014-305131.Wong TY, Ohno-Matsui K, Leveziel N, et al. Myopic
choroidal neovascularisation: current concepts and update
on clinical management. Br J Ophthalmol. 2015, 99(3):
289-296. DOI: 10.1136/bjophthalmol-2014-305131.
10、Kojima A, Ohno-Matsui K, Teramukai S, et al. Factors
associated with the development of chorioretinal atrophy
around choroidal neovascularization in pathologic myopia.
Graefes Arch Clin Exp Ophthalmol. 2004, 242(2): 114-
119. DOI: 10.1007/s00417-003-0803-9.Kojima A, Ohno-Matsui K, Teramukai S, et al. Factors
associated with the development of chorioretinal atrophy
around choroidal neovascularization in pathologic myopia.
Graefes Arch Clin Exp Ophthalmol. 2004, 242(2): 114-
119. DOI: 10.1007/s00417-003-0803-9.
11、Fang Y, Yokoi T, Nagaoka N, et al. Progression of
myopic maculopathy during 18-year follow-up.
Ophthalmology. 2018, 125(6): 863-877. DOI: 10.1016/
j.ophtha.2017.12.005.Fang Y, Yokoi T, Nagaoka N, et al. Progression of
myopic maculopathy during 18-year follow-up.
Ophthalmology. 2018, 125(6): 863-877. DOI: 10.1016/
j.ophtha.2017.12.005.
12、Cheung CMG, Arnold JJ, Holz FG, et al. Myopic choroidal
neovascularization: review, guidance, and consensus
statement on management. Ophthalmology. 2017, 124(11):
1690-1711. DOI: 10.1016/j.ophtha.2017.04.028.Cheung CMG, Arnold JJ, Holz FG, et al. Myopic choroidal
neovascularization: review, guidance, and consensus
statement on management. Ophthalmology. 2017, 124(11):
1690-1711. DOI: 10.1016/j.ophtha.2017.04.028.
13、Yokoi T, Ohno-Matsui K. Diagnosis and treatment of myopic maculopathy. Asia Pac J Ophthalmol. 2018, 7(6):
415-421. DOI: 10.22608/APO.2018290.Yokoi T, Ohno-Matsui K. Diagnosis and treatment of myopic maculopathy. Asia Pac J Ophthalmol. 2018, 7(6):
415-421. DOI: 10.22608/APO.2018290.
14、Bruyère E, Caillaux V, Cohen SY, et al. Spectral-domain
optical coherence tomography of subretinal hyperreflective
exudation in myopic choroidal neovascularization. Am
J Ophthalmol. 2015, 160(4): 749-758.e1. DOI: 10.1016/
j.ajo.2015.07.004.Bruyère E, Caillaux V, Cohen SY, et al. Spectral-domain
optical coherence tomography of subretinal hyperreflective
exudation in myopic choroidal neovascularization. Am
J Ophthalmol. 2015, 160(4): 749-758.e1. DOI: 10.1016/
j.ajo.2015.07.004.
15、Ding X, Zhan Z, Sun L, et al. Retinal pigmental epithelium
elevation and external limiting membrane interruption
in myopic choroidal neovascularization: correlation
with activity. Graefes Arch Clin Exp Ophthalmol. 2018,
256(10): 1831-1837. DOI: 10.1007/s00417-018-4060-3.Ding X, Zhan Z, Sun L, et al. Retinal pigmental epithelium
elevation and external limiting membrane interruption
in myopic choroidal neovascularization: correlation
with activity. Graefes Arch Clin Exp Ophthalmol. 2018,
256(10): 1831-1837. DOI: 10.1007/s00417-018-4060-3.
16、Querques L, Giuffrè C, Corvi F, et al. Optical coherence
tomography angiography of myopic choroidal
neovascularisation. Br J Ophthalmol. 2017, 101(5): 609-
615. DOI: 10.1136/bjophthalmol-2016-309162.Querques L, Giuffrè C, Corvi F, et al. Optical coherence
tomography angiography of myopic choroidal
neovascularisation. Br J Ophthalmol. 2017, 101(5): 609-
615. DOI: 10.1136/bjophthalmol-2016-309162.
17、Li S, Sun L, Zhao X, et al. Assessingtheactivityofmyopic
choroidalneovascularization: comparison between optical
coherence tomography angiography and dye angiography.
Retina. 2020, 40(9): 1757-1764. DOI: 10.1097/
IAE.0000000000002650.Li S, Sun L, Zhao X, et al. Assessingtheactivityofmyopic
choroidalneovascularization: comparison between optical
coherence tomography angiography and dye angiography.
Retina. 2020, 40(9): 1757-1764. DOI: 10.1097/
IAE.0000000000002650.
18、Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic
choroidal neovascularization: a 10-year follow-up.
Ophthalmology. 2003, 110(7): 1297-1305. DOI: 10.1016/
S0161-6420(03)00461-5.Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic
choroidal neovascularization: a 10-year follow-up.
Ophthalmology. 2003, 110(7): 1297-1305. DOI: 10.1016/
S0161-6420(03)00461-5.
19、Hung KC, Wang SW, Hsia Y, et al. Natural course
of the intraretinal hyperreflective sign after macular
haemorrhage absorption in eyes with pathologic myopia.
Acta Ophthalmol. 2020, 98(5): e631-e638. DOI: 10.1111/
aos.14332.Hung KC, Wang SW, Hsia Y, et al. Natural course
of the intraretinal hyperreflective sign after macular
haemorrhage absorption in eyes with pathologic myopia.
Acta Ophthalmol. 2020, 98(5): e631-e638. DOI: 10.1111/
aos.14332.
20、Verteporfin in Photodynamic Therapy Study Group.
Photodynamic therapy of subfoveal choroidal
neovascularization in pathologic myopia with verteporfin.
1-year results of a randomized clinical trial: VIP report no.
1. Ophthalmology. 2001, 108(5): 841-852. DOI: 10.1016/s0161-6420(01)00544-9.Verteporfin in Photodynamic Therapy Study Group.
Photodynamic therapy of subfoveal choroidal
neovascularization in pathologic myopia with verteporfin.
1-year results of a randomized clinical trial: VIP report no.
1. Ophthalmology. 2001, 108(5): 841-852. DOI: 10.1016/s0161-6420(01)00544-9.
21、Ohno-Matsui K, Ikuno Y, Lai TYY, et al. Diagnosis
and treatment guideline for myopic choroidal
neovascularization due to pathologic myopia. Prog
Retin Eye Res. 2018, 63: 92-106. DOI: 10.1016/
j.preteyeres.2017.10.005.Ohno-Matsui K, Ikuno Y, Lai TYY, et al. Diagnosis
and treatment guideline for myopic choroidal
neovascularization due to pathologic myopia. Prog
Retin Eye Res. 2018, 63: 92-106. DOI: 10.1016/
j.preteyeres.2017.10.005.
22、El Matri L, Chebil A, Kort F. Current and emerging
treatment options for myopic choroidal neovascularization.
Clin Ophthalmol, 2015, 9: 733-744. DOI: 10.2147/OPTH.
S49437.El Matri L, Chebil A, Kort F. Current and emerging
treatment options for myopic choroidal neovascularization.
Clin Ophthalmol, 2015, 9: 733-744. DOI: 10.2147/OPTH.
S49437.
23、Lai TYY, Luk FOJ, Lee GKY, et al. Long-term outcome of
intravitreal anti-vascular endothelial growth factor therapy
with bevacizumab or ranibizumab as primary treatment
for subfoveal myopic choroidal neovascularization. Eye.
2012, 26(7): 1004-1011. DOI: 10.1038/eye.2012.97.Lai TYY, Luk FOJ, Lee GKY, et al. Long-term outcome of
intravitreal anti-vascular endothelial growth factor therapy
with bevacizumab or ranibizumab as primary treatment
for subfoveal myopic choroidal neovascularization. Eye.
2012, 26(7): 1004-1011. DOI: 10.1038/eye.2012.97.
24、Wang E, Chen Y. Intravitreal anti-vascular endothelial
growth factor for choroidal neovascularization secondary
to pathologic myopia: systematic review and meta�analysis. Retina. 2013, 33(7): 1375-1392. DOI: 10.1097/
IAE.0b013e31827d260a.Wang E, Chen Y. Intravitreal anti-vascular endothelial
growth factor for choroidal neovascularization secondary
to pathologic myopia: systematic review and meta�analysis. Retina. 2013, 33(7): 1375-1392. DOI: 10.1097/
IAE.0b013e31827d260a.
25、Li S, Ding X, ZhangJ, et al. Two different initial
treatment regimens of ranibizumab in myopic choroidal
neovascularization: 12-Month results from a randomized
controlled study-Response. Clin Exp Ophthalmol. 2019,
47(5): 685-686. DOI: 10.1111/ceo.13507.Li S, Ding X, ZhangJ, et al. Two different initial
treatment regimens of ranibizumab in myopic choroidal
neovascularization: 12-Month results from a randomized
controlled study-Response. Clin Exp Ophthalmol. 2019,
47(5): 685-686. DOI: 10.1111/ceo.13507.
26、Gong B, Bo Y, Zhang P, et al. Efficacy and safety of
different conbercept injection regimens in the treatment
of choroidal neovascularization in pathological myopia: a
retrospective study. Int Ophthalmol. 2023, 43(11): 4079-
4086. DOI: 10.1007/s10792-023-02825-9.Gong B, Bo Y, Zhang P, et al. Efficacy and safety of
different conbercept injection regimens in the treatment
of choroidal neovascularization in pathological myopia: a
retrospective study. Int Ophthalmol. 2023, 43(11): 4079-
4086. DOI: 10.1007/s10792-023-02825-9.
27、Glachs L, Embacher S, Berghold A, et al. Treatment of
myopic choroidal neovascularization: a network meta�analysis and review. Graefes Arch Clin Exp Ophthalmol.
2024, 262(6): 1693-1722. DOI: 10.1007/s00417-023-
06271-2.Glachs L, Embacher S, Berghold A, et al. Treatment of
myopic choroidal neovascularization: a network meta�analysis and review. Graefes Arch Clin Exp Ophthalmol.
2024, 262(6): 1693-1722. DOI: 10.1007/s00417-023-
06271-2.
28、Chen Y, Sharma T, Li X, et al. Ranibizumabversusvertepor
finphotodynamictherapyin Asianpatientswithmyopicchoroi
dalneovascularization: brilliance, a 12-month, randomized,
double-masked study. Retina, 2019, 39(10): 1985-1994.
DOI: 10.1097/IAE.0000000000002292.Chen Y, Sharma T, Li X, et al. Ranibizumabversusvertepor
finphotodynamictherapyin Asianpatientswithmyopicchoroi
dalneovascularization: brilliance, a 12-month, randomized,
double-masked study. Retina, 2019, 39(10): 1985-1994.
DOI: 10.1097/IAE.0000000000002292.
29、Howaidy A, Eldaly ZH. Comparison of structural and
functional outcome of aflibercept versus ranibizumab
in patients with myopic choroidal neovascularization.
Eur J Ophthalmol. 2021, 31(1): 211-217. DOI:
10.1177/1120672119883590.Howaidy A, Eldaly ZH. Comparison of structural and
functional outcome of aflibercept versus ranibizumab
in patients with myopic choroidal neovascularization.
Eur J Ophthalmol. 2021, 31(1): 211-217. DOI:
10.1177/1120672119883590.
30、Gharbiya M, Giustolisi R, Allievi F, et al. Choroidal
neovascularization in pathologic myopia: intravitreal
ranibizumab versus bevacizumab: a randomized controlled
trial. Am J Ophthalmol.2010, 149(3): 458-464.e1. DOI:
10.1016/j.ajo.2009.10.010.Gharbiya M, Giustolisi R, Allievi F, et al. Choroidal
neovascularization in pathologic myopia: intravitreal
ranibizumab versus bevacizumab: a randomized controlled
trial. Am J Ophthalmol.2010, 149(3): 458-464.e1. DOI:
10.1016/j.ajo.2009.10.010.
31、Iacono P, Parodi MB, Papayannis A, et al. Intravitreal
ranibizumab versus bevacizumab for treatment of myopic
choroidal neovascularization. Retina. 2012, 32(8): 1539-
1546. DOI: 10.1097/IAE.0b013e31826956b7.Iacono P, Parodi MB, Papayannis A, et al. Intravitreal
ranibizumab versus bevacizumab for treatment of myopic
choroidal neovascularization. Retina. 2012, 32(8): 1539-
1546. DOI: 10.1097/IAE.0b013e31826956b7.
32、Korol A, Kustryn T, Zadorozhnyy O, etal. Comparison
of efficacy of intravitreal ranibizumab and aflibercept in
eyes with myopic choroidal neovascularization: 24-month
follow-up. J Ocul Pharmacol Ther. 2020, 36(2): 122-125.
DOI: 10.1089/jop.2019.0080.Korol A, Kustryn T, Zadorozhnyy O, etal. Comparison
of efficacy of intravitreal ranibizumab and aflibercept in
eyes with myopic choroidal neovascularization: 24-month
follow-up. J Ocul Pharmacol Ther. 2020, 36(2): 122-125.
DOI: 10.1089/jop.2019.0080.
33、Ikuno Y, Ohno-Matsui K, Wong TY, et al. Intravitreal
a f l i b e r c e p t i n j e c t i o n i n p a t i e n t s w i t h m y o p i c
choroidal neovascularization: the MYRROR study.
Ophthalmology. 2015, 122(6): 1220-1227. DOI: 10.1016/
j.ophtha.2015.01.025.Ikuno Y, Ohno-Matsui K, Wong TY, et al. Intravitreal
a f l i b e r c e p t i n j e c t i o n i n p a t i e n t s w i t h m y o p i c
choroidal neovascularization: the MYRROR study.
Ophthalmology. 2015, 122(6): 1220-1227. DOI: 10.1016/
j.ophtha.2015.01.025.
34、Tan CS, Cheong KX, Lim LW, etal. A randomized trial of
intravitreal bevacizumab vs. ranibizumab for myopic CNV.
Graefes Arch Clin Exp Ophthalmol. 2016, 254(7): 1433-
1434. DOI: 10.1007/s00417-016-3284-3.Tan CS, Cheong KX, Lim LW, etal. A randomized trial of
intravitreal bevacizumab vs. ranibizumab for myopic CNV.
Graefes Arch Clin Exp Ophthalmol. 2016, 254(7): 1433-
1434. DOI: 10.1007/s00417-016-3284-3.
35、Kalogeropoulos D, Rahman N, Afshar F, et al. Punctate
inner choroidopathy: a review of the current diagnostic and therapeutic approaches. Prog Retin Eye Res. 2024, 99:
101235. DOI: 10.1016/j.preteyeres.2023.101235.Kalogeropoulos D, Rahman N, Afshar F, et al. Punctate
inner choroidopathy: a review of the current diagnostic and therapeutic approaches. Prog Retin Eye Res. 2024, 99:
101235. DOI: 10.1016/j.preteyeres.2023.101235.
36、Gerstenblith AT, Thorne JE, Sobrin L, et al. Punctate
inner choroidopathy: a survey analysis of 77 persons.
Ophthalmology. 2007, 114(6): 1201-1204. DOI: 10.1016/
j.ophtha.2006.10.047.Gerstenblith AT, Thorne JE, Sobrin L, et al. Punctate
inner choroidopathy: a survey analysis of 77 persons.
Ophthalmology. 2007, 114(6): 1201-1204. DOI: 10.1016/
j.ophtha.2006.10.047.
37、Essex RW, Wong J, Fraser-Bell S, et al. Punctate inner
choroidopathy: clinical features and outcomes. Arch
Ophthalmol. 2010, 128(8): 982-987. DOI: 10.1001/
archophthalmol.2010.157.Essex RW, Wong J, Fraser-Bell S, et al. Punctate inner
choroidopathy: clinical features and outcomes. Arch
Ophthalmol. 2010, 128(8): 982-987. DOI: 10.1001/
archophthalmol.2010.157.
38、Ahnood D, Madhusudhan S, Tsaloumas MD, et al. Punctate
inner choroidopathy: a review. Surv Ophthalmol, 2017,
62(2): 113-126. DOI: 10.1016/j.survophthal.2016.10.003.Ahnood D, Madhusudhan S, Tsaloumas MD, et al. Punctate
inner choroidopathy: a review. Surv Ophthalmol, 2017,
62(2): 113-126. DOI: 10.1016/j.survophthal.2016.10.003.
39、Amer R, Lois N. Punctate inner choroidopathy. Surv
Ophthalmol. 2011, 56(1): 36-53. DOI: 10.1016/
j.survophthal.2010.03.009.Amer R, Lois N. Punctate inner choroidopathy. Surv
Ophthalmol. 2011, 56(1): 36-53. DOI: 10.1016/
j.survophthal.2010.03.009.
40、Zhang X, Wen F, Zuo C, et al. Clinical features of punctate
inner choroidopathy in Chinese patients. Retina. 2011,
31(8): 1680-1691. DOI: 10.1097/IAE.0b013e31820a67ad.Zhang X, Wen F, Zuo C, et al. Clinical features of punctate
inner choroidopathy in Chinese patients. Retina. 2011,
31(8): 1680-1691. DOI: 10.1097/IAE.0b013e31820a67ad.
41、Patel KH, Birnbaum AD, Tessler HH, et al. Presentation
and outcome of patients with punctate inner choroidopathy
at a tertiary referral center. Retina. 2011, 31(7): 1387-
1391. DOI: 10.1097/IAE.0b013e3182069a8f.Patel KH, Birnbaum AD, Tessler HH, et al. Presentation
and outcome of patients with punctate inner choroidopathy
at a tertiary referral center. Retina. 2011, 31(7): 1387-
1391. DOI: 10.1097/IAE.0b013e3182069a8f.
42、Niederer RL, Gilbert R, Lightman SL, et al. Risk factors
for developing choroidal neovascular membrane
and visual loss in punctate inner choroidopathy.
Ophthalmology. 2018, 125(2): 288-294. DOI: 10.1016/
j.ophtha.2017.09.002.Niederer RL, Gilbert R, Lightman SL, et al. Risk factors
for developing choroidal neovascular membrane
and visual loss in punctate inner choroidopathy.
Ophthalmology. 2018, 125(2): 288-294. DOI: 10.1016/
j.ophtha.2017.09.002.
43、Zhang X, Zuo C, Li M, et al. Spectral-domain optical
coherence tomographic findings at each stage of punctate
inner choroidopathy. Ophthalmology. 2013, 120(12):
2678-2683. DOI: 10.1016/j.ophtha.2013.05.012.Zhang X, Zuo C, Li M, et al. Spectral-domain optical
coherence tomographic findings at each stage of punctate
inner choroidopathy. Ophthalmology. 2013, 120(12):
2678-2683. DOI: 10.1016/j.ophtha.2013.05.012.
44、Spaide RF, Goldberg N, Freund KB. Redefining
multifocal choroiditis and panuveitis and punctate inner
choroidopathy through multimodal imaging. Retina. 2013, 33(7): 1315-1324. DOI: 10.1097/IAE.0b013e318286cc77.Spaide RF, Goldberg N, Freund KB. Redefining
multifocal choroiditis and panuveitis and punctate inner
choroidopathy through multimodal imaging. Retina. 2013, 33(7): 1315-1324. DOI: 10.1097/IAE.0b013e318286cc77.
45、Chen Y, Chen Q, Li X, et al. RPE disruption and hyper-transmission are early signs of secondary CNV with
punctate inner choroidopathy in structure-OCT. BMC
Ophthalmol. 2021, 21(1): 427. DOI: 10.1186/s12886-021-
02197-7.Chen Y, Chen Q, Li X, et al. RPE disruption and hyper-transmission are early signs of secondary CNV with
punctate inner choroidopathy in structure-OCT. BMC
Ophthalmol. 2021, 21(1): 427. DOI: 10.1186/s12886-021-
02197-7.
46、Levison AL, Baynes KM, Lowder CY, et al. Choroidal
neovascularisation on optical coherence tomography
angiography in punctate inner choroidopathy and
multifocal choroiditis. Br J Ophthalmol. 2017, 101(5):
616-622. DOI: 10.1136/bjophthalmol-2016-308806.Levison AL, Baynes KM, Lowder CY, et al. Choroidal
neovascularisation on optical coherence tomography
angiography in punctate inner choroidopathy and
multifocal choroiditis. Br J Ophthalmol. 2017, 101(5):
616-622. DOI: 10.1136/bjophthalmol-2016-308806.
47、Gilbert RM, Niederer RL, Kramer M, et al. Differentiating
multifocal choroiditis and punctate inner choroidopathy:
acluster analysis approach. Am J Ophthalmol. 2020, 213:
244-251. DOI: 10.1016/j.ajo.2020.01.031.Gilbert RM, Niederer RL, Kramer M, et al. Differentiating
multifocal choroiditis and punctate inner choroidopathy:
acluster analysis approach. Am J Ophthalmol. 2020, 213:
244-251. DOI: 10.1016/j.ajo.2020.01.031.
48、Levy J, Shneck M, Klemperer I, et al. Punctate inner
choroidopathy: resolution after oral steroid treatment and
review of the literature. Can J Ophthalmol. 2005, 40(5):
605-608. DOI: 10.1016/S0008-4182(05)80053-5.Levy J, Shneck M, Klemperer I, et al. Punctate inner
choroidopathy: resolution after oral steroid treatment and
review of the literature. Can J Ophthalmol. 2005, 40(5):
605-608. DOI: 10.1016/S0008-4182(05)80053-5.
49、Vienne-Jumeau A, Brézin AP, Seminel M, et al.
Corticosteroids decrease the incidence and activity of
choroidal neovascularization in patients with punctuate
inner choroidopathy or multifocal choroiditis. Ocul
Immunol Inflamm. 2024, 32(5): 602-608. DOI:
10.1080/09273948.2023.2181189.Vienne-Jumeau A, Brézin AP, Seminel M, et al.
Corticosteroids decrease the incidence and activity of
choroidal neovascularization in patients with punctuate
inner choroidopathy or multifocal choroiditis. Ocul
Immunol Inflamm. 2024, 32(5): 602-608. DOI:
10.1080/09273948.2023.2181189.
50、Papasavvas I, Jr Herbort CP. Diagnosis and treatment of
primary inflammatory choriocapillaropathies (PICCPs):
acomprehensive overview. Medicina. 2022, 58(2): 165.
DOI: 10.3390/medicina58020165.Papasavvas I, Jr Herbort CP. Diagnosis and treatment of
primary inflammatory choriocapillaropathies (PICCPs):
acomprehensive overview. Medicina. 2022, 58(2): 165.
DOI: 10.3390/medicina58020165.
51、Chan WM, Lai TYY, Liu DTL, et al. Intravitreal
bevacizumab (avastin) for choroidal neovascularization
secondary to central serous chorioretinopathy, secondary
to punctate inner choroidopathy, or of idiopathic origin.
Am J Ophthalmol. 2007, 143(6): 977-983. DOI: 10.1016/
j.ajo.2007.02.039.Chan WM, Lai TYY, Liu DTL, et al. Intravitreal
bevacizumab (avastin) for choroidal neovascularization
secondary to central serous chorioretinopathy, secondary
to punctate inner choroidopathy, or of idiopathic origin.
Am J Ophthalmol. 2007, 143(6): 977-983. DOI: 10.1016/
j.ajo.2007.02.039.
52、Rouvas A, Petrou P, Douvali M, et al. Intravitreal
ranibizumab for the treatment of inflammatory choroidal
neovascularization. Retina. 2011, 31(5): 871-879. DOI:
10.1097/IAE.0b013e3182003ca8.Rouvas A, Petrou P, Douvali M, et al. Intravitreal
ranibizumab for the treatment of inflammatory choroidal
neovascularization. Retina. 2011, 31(5): 871-879. DOI:
10.1097/IAE.0b013e3182003ca8.
53、Arrevola L, Acero MA, Pera MJ. Two-year
outcome of aflibercept for the treatment of choroidal
neovascularization in punctate inner choroidopathy.
Case Rep Ophthalmol. 2019, 10(1): 24-31. DOI:
10.1159/000496143.Arrevola L, Acero MA, Pera MJ. Two-year
outcome of aflibercept for the treatment of choroidal
neovascularization in punctate inner choroidopathy.
Case Rep Ophthalmol. 2019, 10(1): 24-31. DOI:
10.1159/000496143.
54、Wu W, Li S, Xu H, et al. Treatment of punctate inner
choroidopathy with choroidal neovascularization using
corticosteroid and intravitreal ranibizumab. Biomed Res
Int. 2018, 2018: 1585803. DOI: 10.1155/2018/1585803.Wu W, Li S, Xu H, et al. Treatment of punctate inner
choroidopathy with choroidal neovascularization using
corticosteroid and intravitreal ranibizumab. Biomed Res
Int. 2018, 2018: 1585803. DOI: 10.1155/2018/1585803.
55、Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International
photographic classification and grading system for myopic
maculopathy. Am J Ophthalmol. 2015, 159(5): 877-883.
e7. DOI: 10.1016/j.ajo.2015.01.022.Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International
photographic classification and grading system for myopic
maculopathy. Am J Ophthalmol. 2015, 159(5): 877-883.
e7. DOI: 10.1016/j.ajo.2015.01.022.
56、Klein RM, Curtin BJ. Lacquer crack lesions in pathologic
myopia. Am J Ophthalmol, 1975, 79(3): 386-392. DOI:
10.1016/0002-9394(75)90611-x.Klein RM, Curtin BJ. Lacquer crack lesions in pathologic
myopia. Am J Ophthalmol, 1975, 79(3): 386-392. DOI:
10.1016/0002-9394(75)90611-x.
57、Yan YN, Wang YX, Yang Y, et al. Ten-year progression of
myopic maculopathy: the Beijing eye study 2001-2011.
Ophthalmology. 2018, 125(8): 1253-1263. DOI: 10.1016/
j.ophtha.2018.01.035.Yan YN, Wang YX, Yang Y, et al. Ten-year progression of
myopic maculopathy: the Beijing eye study 2001-2011.
Ophthalmology. 2018, 125(8): 1253-1263. DOI: 10.1016/
j.ophtha.2018.01.035.
58、Coco-Martin RM, Belani-Raju M, de la Fuente-Gomez D,
et al. Progression of myopic maculopathy in a Caucasian
cohort of highly myopic patients with long follow-up: a
multistate analysis. Graefes Arch Clin Exp Ophthalmol.
2021, 259(1): 81-92. DOI: 10.1007/s00417-020-04795-5.Coco-Martin RM, Belani-Raju M, de la Fuente-Gomez D,
et al. Progression of myopic maculopathy in a Caucasian
cohort of highly myopic patients with long follow-up: a
multistate analysis. Graefes Arch Clin Exp Ophthalmol.
2021, 259(1): 81-92. DOI: 10.1007/s00417-020-04795-5.
59、Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term
pattern of progression of myopic maculopathy: a natural
history study. Ophthalmology. 2010, 117(8): 1595-1611,
1611.e1-1611.e4. DOI: 10.1016/j.ophtha.2009.11.003.Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term
pattern of progression of myopic maculopathy: a natural
history study. Ophthalmology. 2010, 117(8): 1595-1611,
1611.e1-1611.e4. DOI: 10.1016/j.ophtha.2009.11.003.
60、Moriyama M, Ohno-Matsui K, Shimada N, et al.
Correlation between visual prognosis and fundus autofluorescence and optical coherence tomographic
findings in highly myopic eyes with submacular
hemorrhage and without choroidal neovascularization.
Retina. 2011, 31(1): 74-80. DOI: 10.1097/IAE.0b013
e3181e91148.Moriyama M, Ohno-Matsui K, Shimada N, et al.
Correlation between visual prognosis and fundus autofluorescence and optical coherence tomographic
findings in highly myopic eyes with submacular
hemorrhage and without choroidal neovascularization.
Retina. 2011, 31(1): 74-80. DOI: 10.1097/IAE.0b013
e3181e91148.
61、Ikuno Y, Sayanagi K, Soga K, et al. Lacquer crack
formation and choroidal neovascularization in pathologic
myopia. Retina. 2008, 28(8): 1124-1131. DOI: 10.1097/
IAE.0b013e318174417a.Ikuno Y, Sayanagi K, Soga K, et al. Lacquer crack
formation and choroidal neovascularization in pathologic
myopia. Retina. 2008, 28(8): 1124-1131. DOI: 10.1097/
IAE.0b013e318174417a.
62、Xu X, Fang Y, Uramoto K, et al. Clinicalfeaturesoflacque
rcracksineyeswithpathologicmyopia. Retina. 2019, 39(7):
1265-1277. DOI: 10.1097/IAE.0000000000002168.Xu X, Fang Y, Uramoto K, et al. Clinicalfeaturesoflacque
rcracksineyeswithpathologicmyopia. Retina. 2019, 39(7):
1265-1277. DOI: 10.1097/IAE.0000000000002168.
63、Shinohara K, Moriyama M, Shimada N, et al.
Myopic stretch lines: linear lesions in fundus of
eyes with pathologic myopia that differ from lacquer
cracks. Retina. 2014, 34(3): 461-469. DOI: 10.1097/
IAE.0b013e3182a6b494.Shinohara K, Moriyama M, Shimada N, et al.
Myopic stretch lines: linear lesions in fundus of
eyes with pathologic myopia that differ from lacquer
cracks. Retina. 2014, 34(3): 461-469. DOI: 10.1097/
IAE.0b013e3182a6b494.
64、Battista M, Sacconi R, Borrelli E, et al. Discerning between
macular hemorrhages due to macular neovascularization
or due to spontaneous bruch’s membrane rupture in
high myopia: acomparative analysis between OCTA and
fluorescein angiography. Ophthalmol Ther. 2022, 11(2):
821-831. DOI: 10.1007/s40123-022-00484-0.Battista M, Sacconi R, Borrelli E, et al. Discerning between
macular hemorrhages due to macular neovascularization
or due to spontaneous bruch’s membrane rupture in
high myopia: acomparative analysis between OCTA and
fluorescein angiography. Ophthalmol Ther. 2022, 11(2):
821-831. DOI: 10.1007/s40123-022-00484-0.
65、Ohno-Matsui K, Ito M, Tokoro T. Subretinal bleeding
without choroidal neovascularization in pathologic myopia.
A sign of new lacquer crack formation. Retina. 1996,
16(3): 196-202. DOI: 10.1097/00006982-199616030-
00003.Ohno-Matsui K, Ito M, Tokoro T. Subretinal bleeding
without choroidal neovascularization in pathologic myopia.
A sign of new lacquer crack formation. Retina. 1996,
16(3): 196-202. DOI: 10.1097/00006982-199616030-
00003.
66、Asai T, Ikuno Y, Nishida K. Macular microstructures and
prognostic factors in myopic subretinal hemorrhages.
Invest Ophthalmol Vis Sci. 2014, 55(1): 226-232. DOI:
10.1167/iovs.13-12658.Asai T, Ikuno Y, Nishida K. Macular microstructures and
prognostic factors in myopic subretinal hemorrhages.
Invest Ophthalmol Vis Sci. 2014, 55(1): 226-232. DOI:
10.1167/iovs.13-12658.
67、Shih YF, Ho TC, Hsiao CK, et al. Visual outcomes for high
myopic patients with or without myopic maculopathy: a 10
year follow up study. Br J Ophthalmol. 2006, 90(5): 546-550. DOI: 10.1136/bjo.2005.081992.Shih YF, Ho TC, Hsiao CK, et al. Visual outcomes for high
myopic patients with or without myopic maculopathy: a 10
year follow up study. Br J Ophthalmol. 2006, 90(5): 546-550. DOI: 10.1136/bjo.2005.081992.
68、Kang HM, Koh HJ. Ocular risk factors for recurrence of
myopic choroidal neovascularization: long-term follow�up study. Retina. 2013, 33(8): 1613-1622. DOI: 10.1097/
IAE.0b013e318285cc24.Kang HM, Koh HJ. Ocular risk factors for recurrence of
myopic choroidal neovascularization: long-term follow�up study. Retina. 2013, 33(8): 1613-1622. DOI: 10.1097/
IAE.0b013e318285cc24.
69、Avila MP, Weiter JJ, Jalkh AE, et al. Natural history of
choroidal neovascularization in degenerative myopia.
Ophthalmology. 1984, 91(12): 1573-1581. DOI: 10.1016/
s0161-6420(84)34116-1.Avila MP, Weiter JJ, Jalkh AE, et al. Natural history of
choroidal neovascularization in degenerative myopia.
Ophthalmology. 1984, 91(12): 1573-1581. DOI: 10.1016/
s0161-6420(84)34116-1.
70、Hung KC, Chen MS, Yang CM, et al. Multimodal imaging
of linear lesions in the fundus of pathologic myopic eyes
with macular lesions. Graefes Arch Clin Exp Ophthalmol.
2018, 256(1): 71-81. DOI: 10.1007/s00417-017-3833-4.Hung KC, Chen MS, Yang CM, et al. Multimodal imaging
of linear lesions in the fundus of pathologic myopic eyes
with macular lesions. Graefes Arch Clin Exp Ophthalmol.
2018, 256(1): 71-81. DOI: 10.1007/s00417-017-3833-4.
71、Neelam K, Ng SMS, Ho EL, et al. Lacquer cracks in
pathological myopia: a clinical review. Eye. 2024,38(15):
2859-2873. DOI: 10.1038/s41433-024-03183-1.Neelam K, Ng SMS, Ho EL, et al. Lacquer cracks in
pathological myopia: a clinical review. Eye. 2024,38(15):
2859-2873. DOI: 10.1038/s41433-024-03183-1.
72、Goldman DR, Vora RA, Reichel E. Traumatic choroidal
rupture with submacular hemorrhage treated with
pneumatic displacement. Retina, 2014, 34(6): 1258-1260.
DOI: 10.1097/IAE.0000000000000019.Goldman DR, Vora RA, Reichel E. Traumatic choroidal
rupture with submacular hemorrhage treated with
pneumatic displacement. Retina, 2014, 34(6): 1258-1260.
DOI: 10.1097/IAE.0000000000000019.
73、Pujari A, Chawla R, Agarwal D, et al. Pathomechanism
of traumatic indirect choroidal rupture. Med Hypotheses.
2019, 124: 64-66. DOI: 10.1016/j.mehy.2019.02.010.Pujari A, Chawla R, Agarwal D, et al. Pathomechanism
of traumatic indirect choroidal rupture. Med Hypotheses.
2019, 124: 64-66. DOI: 10.1016/j.mehy.2019.02.010.
74、Pierro L, Giuffrè C, Rabiolo A, et al. Multimodal imaging
in a patient with traumatic choroidal ruptures. Eur J
Ophthalmol. 2017, 27(6): e175-e178. DOI: 10.5301/
ejo.5001005.Pierro L, Giuffrè C, Rabiolo A, et al. Multimodal imaging
in a patient with traumatic choroidal ruptures. Eur J
Ophthalmol. 2017, 27(6): e175-e178. DOI: 10.5301/
ejo.5001005.
75、Wood CM, Richardson J. Chorioretinal neovascular
membranes complicating contusional eye injuries with
indirect choroidal ruptures. Br J Ophthalmol. 1990, 74(2):
93-96. DOI: 10.1136/bjo.74.2.93.Wood CM, Richardson J. Chorioretinal neovascular
membranes complicating contusional eye injuries with
indirect choroidal ruptures. Br J Ophthalmol. 1990, 74(2):
93-96. DOI: 10.1136/bjo.74.2.93.
76、Ament CS, Zacks DN, Lane AM, et al. Predictors of visual
outcome and choroidal neovascular membrane formation
after traumatic choroidal rupture. Arch Ophthalmol. 2006,
124(7): 957-966. DOI: 10.1001/archopht.124.7.957.Ament CS, Zacks DN, Lane AM, et al. Predictors of visual
outcome and choroidal neovascular membrane formation
after traumatic choroidal rupture. Arch Ophthalmol. 2006,
124(7): 957-966. DOI: 10.1001/archopht.124.7.957.
77、Venkatesh R, Bavaharan B, Yadav NK. Predictors
for choroidal neovascular membrane formation and
visual outcome following blunt ocular trauma. Ther
Adv Ophthalmol. 2019, 11: 2515841419852011. DOI:
10.1177/2515841419852011.Venkatesh R, Bavaharan B, Yadav NK. Predictors
for choroidal neovascular membrane formation and
visual outcome following blunt ocular trauma. Ther
Adv Ophthalmol. 2019, 11: 2515841419852011. DOI:
10.1177/2515841419852011.
78、Russell JF, Albini TA, Berrocal AM, et al. Anti�vascular endothelial growth factor therapy for choroidal
rupture-associated choroidal neovascularization.
Ophthalmol Retina. 2020, 4(2): 226-228. DOI: 10.1016/
j.oret.2019.09.008.Russell JF, Albini TA, Berrocal AM, et al. Anti�vascular endothelial growth factor therapy for choroidal
rupture-associated choroidal neovascularization.
Ophthalmol Retina. 2020, 4(2): 226-228. DOI: 10.1016/
j.oret.2019.09.008.
79、Tervo%20T%2C%20Zetterstr%C3%B6m%20C%2C%20Uusitalo%20R%2C%20et%20al.%20Macular%20%0Ahaemorrhage%20following%20implantation%20of%20a%20posterior%20%0Achamber%20collamer%20lens.%20Acta%20Ophthalmol%20Scand.%202004%2C%20%0A82(2)%3A%20214-215.%20DOI%3A%2010.1111%2Fj.1600-0420.2004.00261.x.Tervo%20T%2C%20Zetterstr%C3%B6m%20C%2C%20Uusitalo%20R%2C%20et%20al.%20Macular%20%0Ahaemorrhage%20following%20implantation%20of%20a%20posterior%20%0Achamber%20collamer%20lens.%20Acta%20Ophthalmol%20Scand.%202004%2C%20%0A82(2)%3A%20214-215.%20DOI%3A%2010.1111%2Fj.1600-0420.2004.00261.x.
80、Principe AH, Lin DY, Small KW, et al. Macular
hemorrhage after laser in situ keratomileusis (LASIK) with
femtosecond laser flap creation. Am J Ophthalmol.2004,
138(4): 657-659. DOI: 10.1016/j.ajo.2004.04.030.Principe AH, Lin DY, Small KW, et al. Macular
hemorrhage after laser in situ keratomileusis (LASIK) with
femtosecond laser flap creation. Am J Ophthalmol.2004,
138(4): 657-659. DOI: 10.1016/j.ajo.2004.04.030.
81、Loewenstein A, Lipshitz I, Varssano D, et al. Macular
hemorrhage after excimer laser photorefractive
keratectomy. J Cataract Refract Surg. 1997, 23(5): 808-
810. DOI: 10.1016/s0886-3350(97)80297-0.Loewenstein A, Lipshitz I, Varssano D, et al. Macular
hemorrhage after excimer laser photorefractive
keratectomy. J Cataract Refract Surg. 1997, 23(5): 808-
810. DOI: 10.1016/s0886-3350(97)80297-0.
82、Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet.
2012, 379(9827): 1739-1748. DOI: 10.1016/S0140-
6736(12)60272-4.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet.
2012, 379(9827): 1739-1748. DOI: 10.1016/S0140-
6736(12)60272-4.
83、Zhang XJ, Chen XN, Tang FY, et al. Pathogenesis of
myopic choroidal neovascularization: a systematic review
and meta-analysis. Surv Ophthalmol. 2023, 68(6): 1011-
1026. DOI: 10.1016/j.survophthal.2023.07.006.Zhang XJ, Chen XN, Tang FY, et al. Pathogenesis of
myopic choroidal neovascularization: a systematic review
and meta-analysis. Surv Ophthalmol. 2023, 68(6): 1011-
1026. DOI: 10.1016/j.survophthal.2023.07.006.
84、Neelam K, Cheung CMG, Ohno-Matsui K, et al.
Choroidal neovascularization in pathological myopia.
Prog Retin Eye Res. 2012, 31(5): 495-525. DOI: 10.1016/
j.preteyeres.2012.04.001.Neelam K, Cheung CMG, Ohno-Matsui K, et al.
Choroidal neovascularization in pathological myopia.
Prog Retin Eye Res. 2012, 31(5): 495-525. DOI: 10.1016/
j.preteyeres.2012.04.001.
85、Ishida T, Watanabe T, Yokoi T, et al. Possible
connection of short posterior ciliary arteries to choroidal neovascularisations in eyes with pathologic myopia. Br
J Ophthalmol. 2019, 103(4): 457-462. DOI: 10.1136/
bjophthalmol-2018-312015.Ishida T, Watanabe T, Yokoi T, et al. Possible
connection of short posterior ciliary arteries to choroidal neovascularisations in eyes with pathologic myopia. Br
J Ophthalmol. 2019, 103(4): 457-462. DOI: 10.1136/
bjophthalmol-2018-312015.
86、Querques G, Corvi F, Balaratnasingam C, et al. Lacquer
cracks and perforating scleral vessels in pathologic myopia:
apossible causal relationship. Am J Ophthalmol. 2015,
160(4): 759-766.e2. DOI: 10.1016/j.ajo.2015.07.017.Querques G, Corvi F, Balaratnasingam C, et al. Lacquer
cracks and perforating scleral vessels in pathologic myopia:
apossible causal relationship. Am J Ophthalmol. 2015,
160(4): 759-766.e2. DOI: 10.1016/j.ajo.2015.07.017.
87、Wang T, Lian P, Zhan J, et al. The landscape of
angiogenesis and inflammatory factors in eyes with
myopic choroidal neovascularization before and after
anti-VEGF injection. Cytokine. 2024, 179: 156640. DOI:
10.1016/j.cyto.2024.156640.Wang T, Lian P, Zhan J, et al. The landscape of
angiogenesis and inflammatory factors in eyes with
myopic choroidal neovascularization before and after
anti-VEGF injection. Cytokine. 2024, 179: 156640. DOI:
10.1016/j.cyto.2024.156640.
88、Daruich A, Matet A, Moulin A, et al. Mechanisms of
macular edema: beyond the surface. Prog Retin Eye Res.
2018, 63: 20-68. DOI: 10.1016/j.preteyeres.2017.10.006.Daruich A, Matet A, Moulin A, et al. Mechanisms of
macular edema: beyond the surface. Prog Retin Eye Res.
2018, 63: 20-68. DOI: 10.1016/j.preteyeres.2017.10.006.
89、Giuffrè C, Querques L, Carnevali A, et al. Choroidal
neovascularization and coincident perforating scleral
vessels in pathologic myopia. Eur J Ophthalmol. 2017,
27(2): e39-e45. DOI: 10.5301/ejo.5000875.Giuffrè C, Querques L, Carnevali A, et al. Choroidal
neovascularization and coincident perforating scleral
vessels in pathologic myopia. Eur J Ophthalmol. 2017,
27(2): e39-e45. DOI: 10.5301/ejo.5000875.
90、Wakabayashi T, Ikuno Y. Choroidal filling delay in
choroidal neovascularisation due to pathological myopia.
Br J Ophthalmol. 2010, 94(5): 611-615. DOI: 10.1136/
bjo.2009.163535.Wakabayashi T, Ikuno Y. Choroidal filling delay in
choroidal neovascularisation due to pathological myopia.
Br J Ophthalmol. 2010, 94(5): 611-615. DOI: 10.1136/
bjo.2009.163535.
91、Leveziel N, Yu Y, Reynolds R, et al. Genetic factors for
choroidal neovascularization associated with high myopia.
Invest Ophthalmol Vis Sci. 2012, 53(8): 5004-5009. DOI:
10.1167/iovs.12-9538.Leveziel N, Yu Y, Reynolds R, et al. Genetic factors for
choroidal neovascularization associated with high myopia.
Invest Ophthalmol Vis Sci. 2012, 53(8): 5004-5009. DOI:
10.1167/iovs.12-9538.
92、Miyake M, Yamashiro K, Nakanishi H, et al. Evaluation of pigment epithelium-derived factor and complement factor I
polymorphisms as a cause of choroidal neovascularization
in highly myopic eyes. Invest Ophthalmol Vis Sci. 2013,
54(6): 4208-4212. DOI: 10.1167/iovs.13-12280.Miyake M, Yamashiro K, Nakanishi H, et al. Evaluation of pigment epithelium-derived factor and complement factor I
polymorphisms as a cause of choroidal neovascularization
in highly myopic eyes. Invest Ophthalmol Vis Sci. 2013,
54(6): 4208-4212. DOI: 10.1167/iovs.13-12280.
93、Akagi-Kurashige Y, Kumagai K, Yamashiro K, et al.
Vascular endothelial growth factor gene polymorphisms
and choroidal neovascularization in highly myopic eyes.
Invest Ophthalmol Vis Sci. 2012, 53(4): 2349-2353. DOI:
10.1167/iovs.11-9405.Akagi-Kurashige Y, Kumagai K, Yamashiro K, et al.
Vascular endothelial growth factor gene polymorphisms
and choroidal neovascularization in highly myopic eyes.
Invest Ophthalmol Vis Sci. 2012, 53(4): 2349-2353. DOI:
10.1167/iovs.11-9405.
94、Kalogeropoulos D, Sung VC, Paschopoulos M, et al. The
physiologic and pathologic effects of pregnancy on the
human visual system. J Obstet Gynaecol. 2019, 39(8):
1037-1048. DOI: 10.1080/01443615.2019.1584891.Kalogeropoulos D, Sung VC, Paschopoulos M, et al. The
physiologic and pathologic effects of pregnancy on the
human visual system. J Obstet Gynaecol. 2019, 39(8):
1037-1048. DOI: 10.1080/01443615.2019.1584891.
95、Lygnos MC, Pappa KI, Papadaki HA, et al. Changes in
maternal plasma levels of VEGF, bFGF, TGF-beta1, ET-1
and sKL during uncomplicated pregnancy, hypertensive
pregnancy and gestational diabetes. In Vivo. 2006, 20(1):
157-163.Lygnos MC, Pappa KI, Papadaki HA, et al. Changes in
maternal plasma levels of VEGF, bFGF, TGF-beta1, ET-1
and sKL during uncomplicated pregnancy, hypertensive
pregnancy and gestational diabetes. In Vivo. 2006, 20(1):
157-163.
96、Krauss T, Pauer HU, Augustin HG. Prospective analysis
of placenta growth factor (PlGF) concentrations in the
plasma of women with normal pregnancy and pregnancies
complicated by preeclampsia. Hypertens Pregnancy. 2004,
23(1): 101-111. DOI: 10.1081/PRG-120028286.Krauss T, Pauer HU, Augustin HG. Prospective analysis
of placenta growth factor (PlGF) concentrations in the
plasma of women with normal pregnancy and pregnancies
complicated by preeclampsia. Hypertens Pregnancy. 2004,
23(1): 101-111. DOI: 10.1081/PRG-120028286.
97、DwivediV, PandeyN. Flare up of choroiditis and
choroidal neovasculazation associated with punctate
inner choroidopathy during early pregnancy. Indian J
Ophthalmol.2012, 60(4): 340;authorreply341. DOI:
10.4103/0301-4738.98738.DwivediV, PandeyN. Flare up of choroiditis and
choroidal neovasculazation associated with punctate
inner choroidopathy during early pregnancy. Indian J
Ophthalmol.2012, 60(4): 340;authorreply341. DOI:
10.4103/0301-4738.98738.