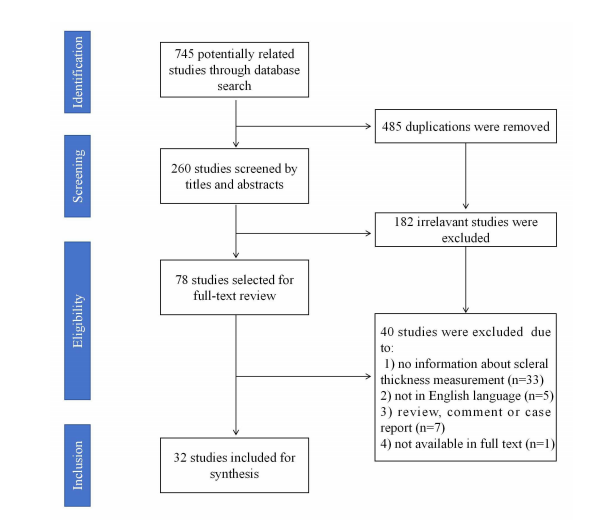
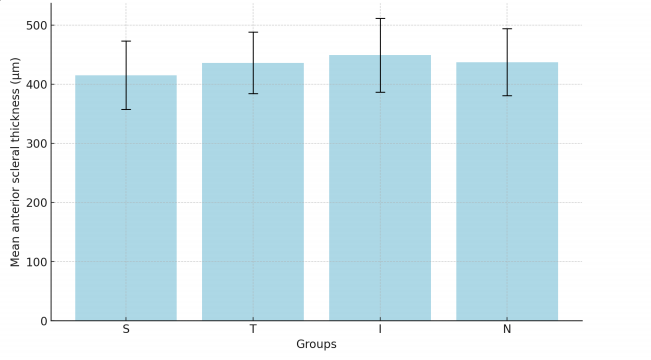
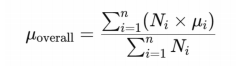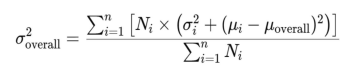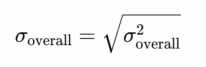1、Boote C, Sigal lA, Grytz R, et al. Scleral structure andbiomechanics. Prog Retin Eye Res.2020,74:100773DOI: 10.1016/j.preteyeres.2019.100773.Boote C, Sigal lA, Grytz R, et al. Scleral structure andbiomechanics. Prog Retin Eye Res.2020,74:100773DOI: 10.1016/j.preteyeres.2019.100773.
2、Kommula H, Murthy Sl, Loomba A, et al. Scleral thicknessin normal Indian eyes measured using spectral domainanterior segment optical coherence tomography, Indian JOphthalmol.2023,71(5):1833-1836,DOI: 10.4103/ijo.IJ0_2046 22.Kommula H, Murthy Sl, Loomba A, et al. Scleral thicknessin normal Indian eyes measured using spectral domainanterior segment optical coherence tomography, Indian JOphthalmol.2023,71(5):1833-1836,DOI: 10.4103/ijo.IJ0_2046 22.
3、Pekel G, Yagci R, Acer S, et al. Comparison of corneallayers and anterior selera in emmetropic and myopieeyes.Cornea.2015,34(7):786-790.DOI: 10.1097/IC0.0000000000000422.Pekel G, Yagci R, Acer S, et al. Comparison of corneallayers and anterior selera in emmetropic and myopieeyes.Cornea.2015,34(7):786-790.DOI: 10.1097/IC0.0000000000000422.
4、Imanaga N, Terao N, Nakamine S, et al. Scleral thicknessin central serous chorioretinopathy, Ophthalmol Retina.2021,5(3):285-291.DOI: 10.1016/i.oret.2020.07.011.Imanaga N, Terao N, Nakamine S, et al. Scleral thicknessin central serous chorioretinopathy, Ophthalmol Retina.2021,5(3):285-291.DOI: 10.1016/i.oret.2020.07.011.
5、Nolan W. Anterior segment imaging: ultrasoundbiomicroscopy and anterior segment optical coherencetomography. Curr Opin Ophthalmol. 2008, 19(2):115-121.DO1: 10.1097/CU.0b013e3282f40bba.Nolan W. Anterior segment imaging: ultrasoundbiomicroscopy and anterior segment optical coherencetomography. Curr Opin Ophthalmol. 2008, 19(2):115-121.DO1: 10.1097/CU.0b013e3282f40bba.
6、Lim SH, Clinical applications of anterior segment opticalcoherence tomography. J Ophthalmol. 2015,2015: 605729DOI:10.1155/2015/605729.Lim SH, Clinical applications of anterior segment opticalcoherence tomography. J Ophthalmol. 2015,2015: 605729DOI:10.1155/2015/605729.
7、Yild1z%20MB%2C%20Bola%C3%A7%20R.Is%20keratoconus%20more%20than%20just%20acorneal%20disease%3F%20Cornea.2024%2C43(3)%3A360-364.%20DOI%3A10.1097%2FC0.0000000000003366.Yild1z%20MB%2C%20Bola%C3%A7%20R.Is%20keratoconus%20more%20than%20just%20acorneal%20disease%3F%20Cornea.2024%2C43(3)%3A360-364.%20DOI%3A10.1097%2FC0.0000000000003366.
8、Gogola A, Jan NJ, Brazile B, et al. Spatial patterns andage-related changes of the collagen crimp in the humancornea and sclera, Invest Ophthalmol Vis Sci. 2018, 59(7):2987-2998.DOI:10.1167/0vs.17-23474.Gogola A, Jan NJ, Brazile B, et al. Spatial patterns andage-related changes of the collagen crimp in the humancornea and sclera, Invest Ophthalmol Vis Sci. 2018, 59(7):2987-2998.DOI:10.1167/0vs.17-23474.
9、Zhou N, Yang L,Xu X,et al. Uveal effusion syndrome:clinical characteristics, outcome of surgical treatment, andhistopathological examination of the sclera, Front Med.2022,9:785444.DOI:10.3389/fmed.2022.785444Zhou N, Yang L,Xu X,et al. Uveal effusion syndrome:clinical characteristics, outcome of surgical treatment, andhistopathological examination of the sclera, Front Med.2022,9:785444.DOI:10.3389/fmed.2022.785444
10、Fernández-Vigo Jl, Shi H, Burgos-Blasco B, et al. Anteriorscleral thickness dimensions by swept-souree opticalcoherence tomography. Clin Exp Optom,2022,105(1):13-19.DOI:10.1080/08164622.2021.1924629Fernández-Vigo Jl, Shi H, Burgos-Blasco B, et al. Anteriorscleral thickness dimensions by swept-souree opticalcoherence tomography. Clin Exp Optom,2022,105(1):13-19.DOI:10.1080/08164622.2021.1924629
11、Ebneter%20A%2C%20H%C3%A4ner%20NU%2C%20Zinkernagel%20MS.%20Metrics%20of%20the%20normal%20anterior%20sclera%3A%20imaging%20with%20optical%20coherencetomography%2C%20Graefes%20Arch%20Clin%20Exp%20Ophthalmol.%202015%2C253(9)%3A1575-1580.DOI%3A10.1007%2Fs00417-015-3072-5.Ebneter%20A%2C%20H%C3%A4ner%20NU%2C%20Zinkernagel%20MS.%20Metrics%20of%20the%20normal%20anterior%20sclera%3A%20imaging%20with%20optical%20coherencetomography%2C%20Graefes%20Arch%20Clin%20Exp%20Ophthalmol.%202015%2C253(9)%3A1575-1580.DOI%3A10.1007%2Fs00417-015-3072-5.
12、Buckhurst HD, Gilmartin B, Cubbidge RP, et al. Measurement ofscleral thickness in humans using anteriorsegment optical coherent tomography. PLoS One. 2015,10(7):e0132902.DOl: 10.1371/jounal.pone.0132902.Buckhurst HD, Gilmartin B, Cubbidge RP, et al. Measurement ofscleral thickness in humans using anteriorsegment optical coherent tomography. PLoS One. 2015,10(7):e0132902.DOl: 10.1371/jounal.pone.0132902.
13、Teeuw GJ, Vergouwen DPC, Ramdas WD, et al.Assessment of conjunctival, episcleral and scleralthickness in healthy individuals using anterior segmentoptical coherence tomography. Acta Ophthalmol. 2024,102(5):573-580.DOl: 10.111l/aos.16606.Teeuw GJ, Vergouwen DPC, Ramdas WD, et al.Assessment of conjunctival, episcleral and scleralthickness in healthy individuals using anterior segmentoptical coherence tomography. Acta Ophthalmol. 2024,102(5):573-580.DOl: 10.111l/aos.16606.
14、Read SA,Alonso-Caneiro D, Free KA, et al. Diurnalvariation of anterior scleral and conjunctival thickness.Ophthalmic Physiol Opt.2016,36(3):279-289.DO1:10.1111/opo.12288.Read SA,Alonso-Caneiro D, Free KA, et al. Diurnalvariation of anterior scleral and conjunctival thickness.Ophthalmic Physiol Opt.2016,36(3):279-289.DO1:10.1111/opo.12288.
15、Fern%C3%83%C3%A1ndez-Vigo%20I%2CFern%C3%A1ndez-Arag%C3%B3n%20S%2CBurgos-Blasco%20B%2C%20et%20al.%20Comparison%20in%20conjunetival-Tenon's%20capsulethickness%2C%20anterior%20scleral%20thickness%20and%20ciliary%20muscledimensions%20between%20Caucasians%20and%20Hispanic%20by%20opticalcoherence%20tomography.%20Int%20Ophthalmol.%202023%2C%2043(11)%3A3969-3977.DOI%3A10.1007%2Fs10792-023-02798-9.Fern%C3%83%C3%A1ndez-Vigo%20I%2CFern%C3%A1ndez-Arag%C3%B3n%20S%2CBurgos-Blasco%20B%2C%20et%20al.%20Comparison%20in%20conjunetival-Tenon's%20capsulethickness%2C%20anterior%20scleral%20thickness%20and%20ciliary%20muscledimensions%20between%20Caucasians%20and%20Hispanic%20by%20opticalcoherence%20tomography.%20Int%20Ophthalmol.%202023%2C%2043(11)%3A3969-3977.DOI%3A10.1007%2Fs10792-023-02798-9.
16、Hau SC, Devarajan K, Ang M. Anterior segment opticalcoherence tomography angiography and optical coherencetomography in the evaluation of episcleritis and scleritis.Ocul Immunol Inflamm.2021,29(2):362-369.DOI:10.1080/09273948.2019.1682617.Hau SC, Devarajan K, Ang M. Anterior segment opticalcoherence tomography angiography and optical coherencetomography in the evaluation of episcleritis and scleritis.Ocul Immunol Inflamm.2021,29(2):362-369.DOI:10.1080/09273948.2019.1682617.
17、Dhakal R, Vupparaboina KK, Verkicharla PK. Anteriorsclera undergoes thinning with increasing degree ofmyopia. Invest Ophthalmol Vis Sci. 2020, 61(4): 6. DOI:10.1167/iovs.61.4.6.Dhakal R, Vupparaboina KK, Verkicharla PK. Anteriorsclera undergoes thinning with increasing degree ofmyopia. Invest Ophthalmol Vis Sci. 2020, 61(4): 6. DOI:10.1167/iovs.61.4.6.
18、Sung MS, Ji YS, Moon HS, et al. Anterior scleralthickness in myopic eyes and its association with ocularparameters.Ophthalmic Res.2021,64(4):567-576.DO1:10.1159/000512396.Sung MS, Ji YS, Moon HS, et al. Anterior scleralthickness in myopic eyes and its association with ocularparameters.Ophthalmic Res.2021,64(4):567-576.DO1:10.1159/000512396.
19、Li M, Luo Z, Yan X, et al. The anterior segment biometriesin high myopia eyes. Ophthalmic Res. 2023, 66(1): 75-85DOI: 10.1159/000526280.Li M, Luo Z, Yan X, et al. The anterior segment biometriesin high myopia eyes. Ophthalmic Res. 2023, 66(1): 75-85DOI: 10.1159/000526280.
20、Schlatter B, Beck M, Frueh BE, et al. Evaluation of scleraland corneal thickness in keratoconus patients. J CataractRefract Surg.2015,41(5):1073-1080.DOI: 10.1016/j.jcrs.2014.08.035.Schlatter B, Beck M, Frueh BE, et al. Evaluation of scleraland corneal thickness in keratoconus patients. J CataractRefract Surg.2015,41(5):1073-1080.DOI: 10.1016/j.jcrs.2014.08.035.
21、Lee Yl, Lee YI, Lee JY, et al. A pilot study of scleral thickness in central serous chorioretinopathy using anteriorsegment optical coherence tomography. Sci Rep. 2021,11(1):5872.DOI:10.1038/s41598-021-85229-y.Lee Yl, Lee YI, Lee JY, et al. A pilot study of scleral thickness in central serous chorioretinopathy using anteriorsegment optical coherence tomography. Sci Rep. 2021,11(1):5872.DOI:10.1038/s41598-021-85229-y.
22、Mohapatra T, Trehan HS, Kurumkattil R, et al.Anterior scleral thickness in patients of central serouschorioretinopathy:a Case-control study. Oman JOphthalmol.2022,16(1):12-17.DOI: 10.4103/ojo. ojo_3_22.Mohapatra T, Trehan HS, Kurumkattil R, et al.Anterior scleral thickness in patients of central serouschorioretinopathy:a Case-control study. Oman JOphthalmol.2022,16(1):12-17.DOI: 10.4103/ojo. ojo_3_22.
23、Aichi T, Terao N, lmanaga N, et al. Scleral thickness inthe fellow eyes of patients with unilateral eentral serouschorioretinopathy,Retina.2023,43(9):1573-1578.DOI:
10.1097/AE.0000000000003850.Aichi T, Terao N, lmanaga N, et al. Scleral thickness inthe fellow eyes of patients with unilateral eentral serouschorioretinopathy,Retina.2023,43(9):1573-1578.DOI:
10.1097/AE.0000000000003850.
24、Wang Y, Wei Sim F, Patrick Wang MA, et al. Changesin scleral thickness following repeated anti-vascularendothelial growth factor injections. J Ophthalmic Vis Res.2022,17(2):196-201.DO1:10.18502/jovr.v17i2.10790.Wang Y, Wei Sim F, Patrick Wang MA, et al. Changesin scleral thickness following repeated anti-vascularendothelial growth factor injections. J Ophthalmic Vis Res.2022,17(2):196-201.DO1:10.18502/jovr.v17i2.10790.
25、Zinkernagel MS, Schorno P, Ebneter A, et al. Scleralthinning after repeated intravitreal injections ofantivascular endothelial growth factor agents in the samequadrant, Invest Ophthalmol Vis Sci. 2015, 56(3):1894-1900.DOI: 10.1167/iovs.14-16204.Zinkernagel MS, Schorno P, Ebneter A, et al. Scleralthinning after repeated intravitreal injections ofantivascular endothelial growth factor agents in the samequadrant, Invest Ophthalmol Vis Sci. 2015, 56(3):1894-1900.DOI: 10.1167/iovs.14-16204.
26、Park JH, Yoo C, Chung HW, et al, Efect of prostaglandinanalogues on anterior scleral thickness and cornealthickness in patients with primary open-angle glaucoma.Sci Rep.2021,11(1):11098.DOI: 10.1038/s41598-021-90696-4.Park JH, Yoo C, Chung HW, et al, Efect of prostaglandinanalogues on anterior scleral thickness and cornealthickness in patients with primary open-angle glaucoma.Sci Rep.2021,11(1):11098.DOI: 10.1038/s41598-021-90696-4.
27、Korkmaz I, Esen Baris M, Guven Yilmaz S, et al. Efectof cycloplegia on anterior segment structures and scleralthickness in emmetropie eyes.I Ocul Pharmacol Ther.2023,39(10):699-704.DOI: 10.1089/jop.2023.0039.Korkmaz I, Esen Baris M, Guven Yilmaz S, et al. Efectof cycloplegia on anterior segment structures and scleralthickness in emmetropie eyes.I Ocul Pharmacol Ther.2023,39(10):699-704.DOI: 10.1089/jop.2023.0039.
28、Adiyeke SK, Kutlu N, Aytogan H, et al. Thicknesses ofsclera and lamina cribrosa in patients with central retinalvein occlusion.Retina.2020.40(10):2050-2054.DOI:10.1097/AE.0000000000002712.Adiyeke SK, Kutlu N, Aytogan H, et al. Thicknesses ofsclera and lamina cribrosa in patients with central retinalvein occlusion.Retina.2020.40(10):2050-2054.DOI:10.1097/AE.0000000000002712.
29、Korkmaz I, Degirmenci C, Selver OB, et al. Evaluationof scleral thickness in patients with Fuchs endothelialdystrophy. Graefes Arch Clin Exp Ophthalmol. 2023,261(10):2883-2889.DOI:10.1007/s00417-023-06107-z.Korkmaz I, Degirmenci C, Selver OB, et al. Evaluationof scleral thickness in patients with Fuchs endothelialdystrophy. Graefes Arch Clin Exp Ophthalmol. 2023,261(10):2883-2889.DOI:10.1007/s00417-023-06107-z.
30、Kaya H,Karasu U,Martin C,et al. Measurements ofscleral thickness and corneal optic densitometry in patientswith systemic lupus erythematosus. Medicine. 2020,99(31):e21467.DOI: 10.1097/MD.0000000000021467.Kaya H,Karasu U,Martin C,et al. Measurements ofscleral thickness and corneal optic densitometry in patientswith systemic lupus erythematosus. Medicine. 2020,99(31):e21467.DOI: 10.1097/MD.0000000000021467.
31、Fern%C2%A1ndez-Vigo%20Jl%2C%20Shi%20H%2C%20Burgos-Blasco%20B%2C%20et%20al.%20Impactof%20age%2C%20sex%20and%20refractive%20error%20on%20conjunctival%20andTenon's%20capsule%20thickness%20dimensions%20by%20swept-sourceoptical%20coherenee%20tomography%20in%20a%20large%20population%2C%20IntOphthalmol.2021%2C41(11)%3A3687-3698.DOI%3A%2010.1007%2Fs10792-021-01928-5.Fern%C2%A1ndez-Vigo%20Jl%2C%20Shi%20H%2C%20Burgos-Blasco%20B%2C%20et%20al.%20Impactof%20age%2C%20sex%20and%20refractive%20error%20on%20conjunctival%20andTenon's%20capsule%20thickness%20dimensions%20by%20swept-sourceoptical%20coherenee%20tomography%20in%20a%20large%20population%2C%20IntOphthalmol.2021%2C41(11)%3A3687-3698.DOI%3A%2010.1007%2Fs10792-021-01928-5.
32、Burguera-Giménez N, Diez-Ajenjo MA, Burguera N, etal. Anterior scleral thickness profle in keratoconus. Life.2023,13(11):2223.DOl: 10.3390/life13112223.Burguera-Giménez N, Diez-Ajenjo MA, Burguera N, etal. Anterior scleral thickness profle in keratoconus. Life.2023,13(11):2223.DOl: 10.3390/life13112223.
33、Sun Y, Sha Y, Yang J, et al. Collagen is crucial targetprotein for scleral remodeling and biomechanical changein myopia progression and control. Heliyon. 2024,10(15):e35313.DOl: 10.1016/j.heliyon.2024.e35313.Sun Y, Sha Y, Yang J, et al. Collagen is crucial targetprotein for scleral remodeling and biomechanical changein myopia progression and control. Heliyon. 2024,10(15):e35313.DOl: 10.1016/j.heliyon.2024.e35313.
34、KhalafAllah MT, Fuchs PA, Nugen F, et al. Heterogenousthinning of peripapillary tissues occurs early during highmyopia development in juvenile tree shrews. Exp Eye Res.2024,240:109824.DOI:10.1016/i.exer.2024.109824.KhalafAllah MT, Fuchs PA, Nugen F, et al. Heterogenousthinning of peripapillary tissues occurs early during highmyopia development in juvenile tree shrews. Exp Eye Res.2024,240:109824.DOI:10.1016/i.exer.2024.109824.
35、BenAbderrahim K.Optical coherence tomography versus ophthalmic examination findings in the management ofanterior scleritis: a prospective study, J Fr Ophtalmol.2022,45(1):40-46.DOI: 10.1016j.jfo.2021.07.009.BenAbderrahim K.Optical coherence tomography versus ophthalmic examination findings in the management ofanterior scleritis: a prospective study, J Fr Ophtalmol.2022,45(1):40-46.DOI: 10.1016j.jfo.2021.07.009.
36、Imanaga N, Terao N, Sawaguchi S, et al. Clinicalfactors related to loculation of fluid in central serouschorioretinopathy.Am J Ophthalmol.2022,235:197-203.DOl: 10.1016/j.ajo.2021.09.009.Imanaga N, Terao N, Sawaguchi S, et al. Clinicalfactors related to loculation of fluid in central serouschorioretinopathy.Am J Ophthalmol.2022,235:197-203.DOl: 10.1016/j.ajo.2021.09.009.