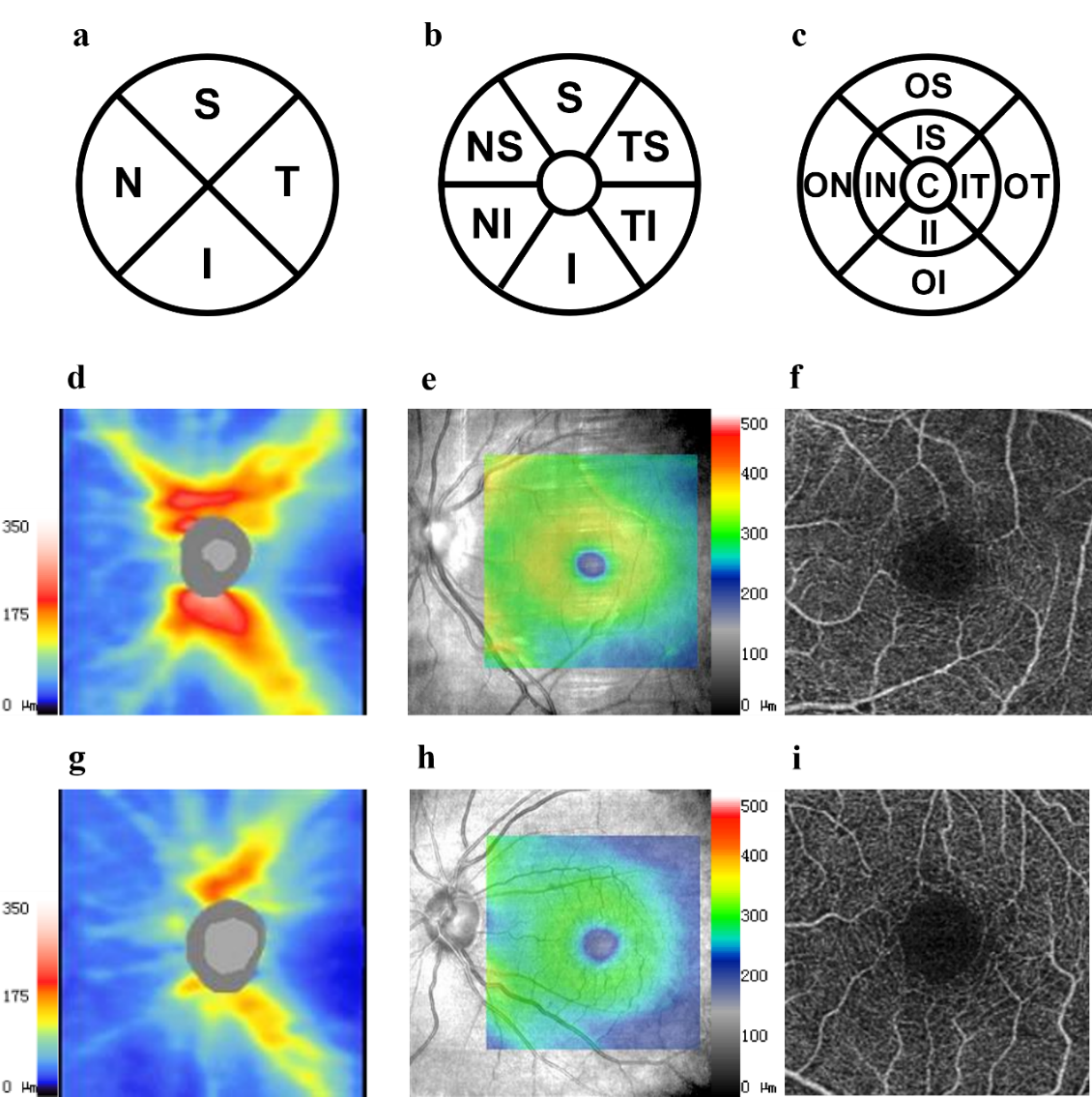
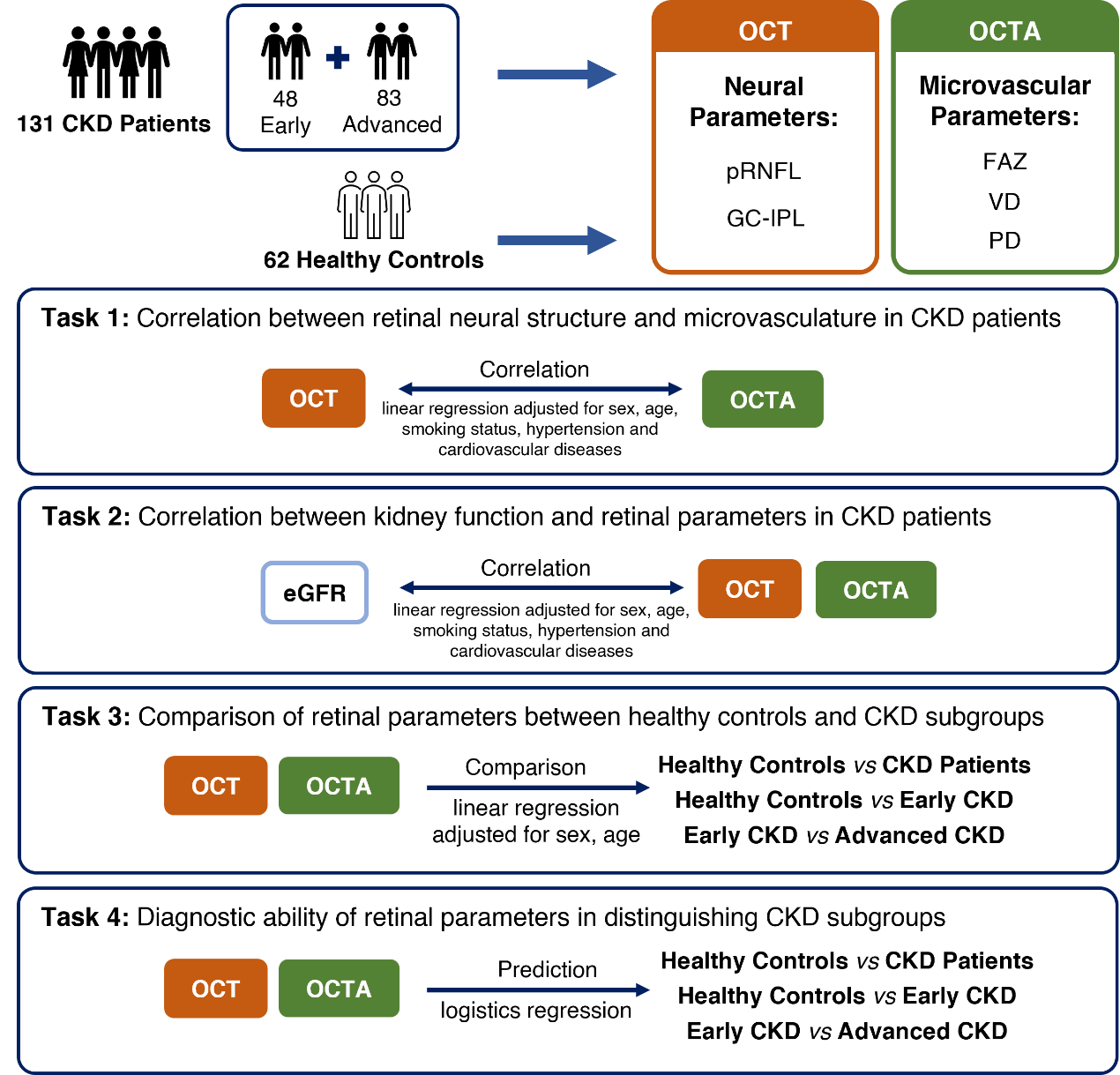
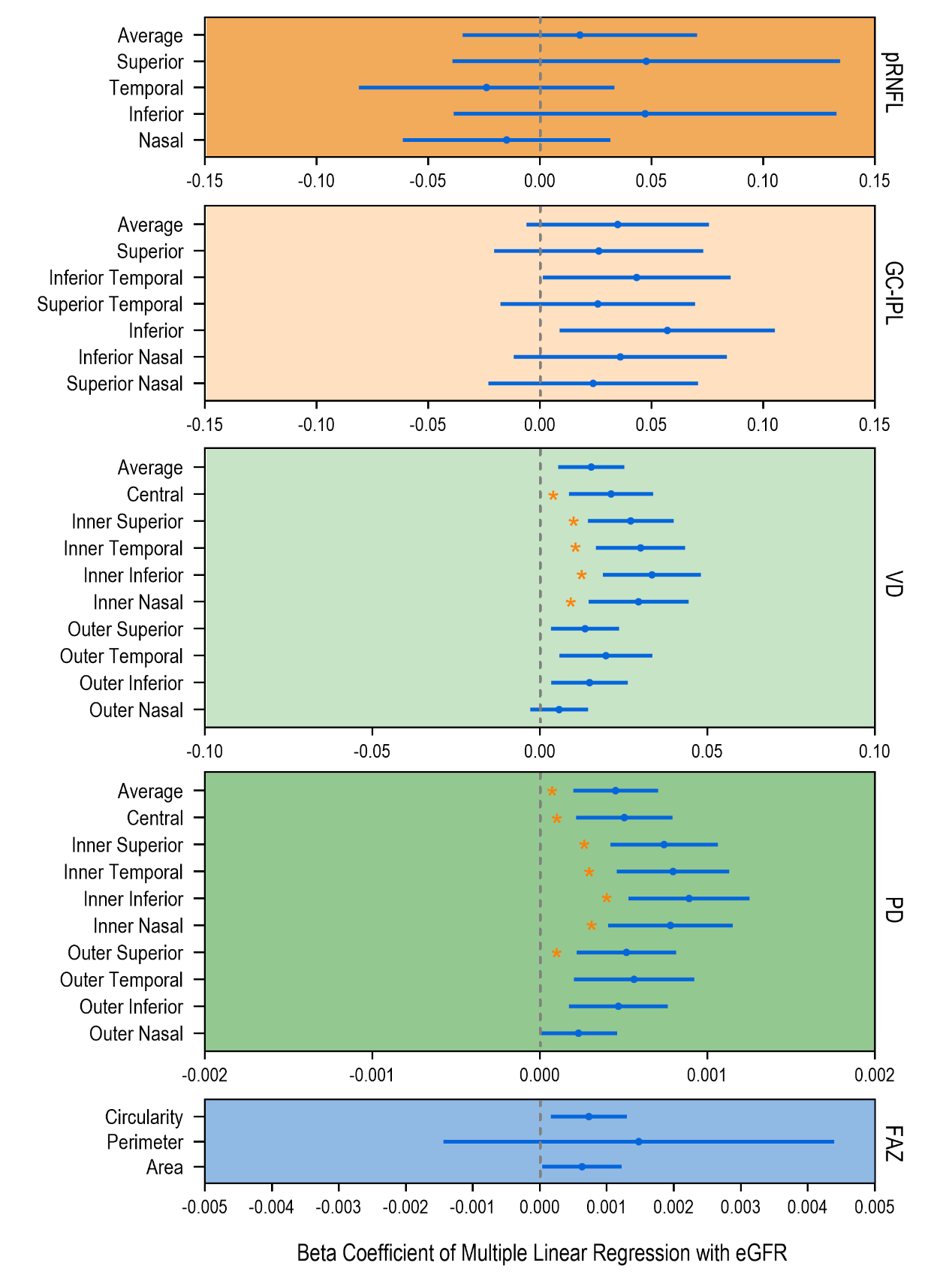
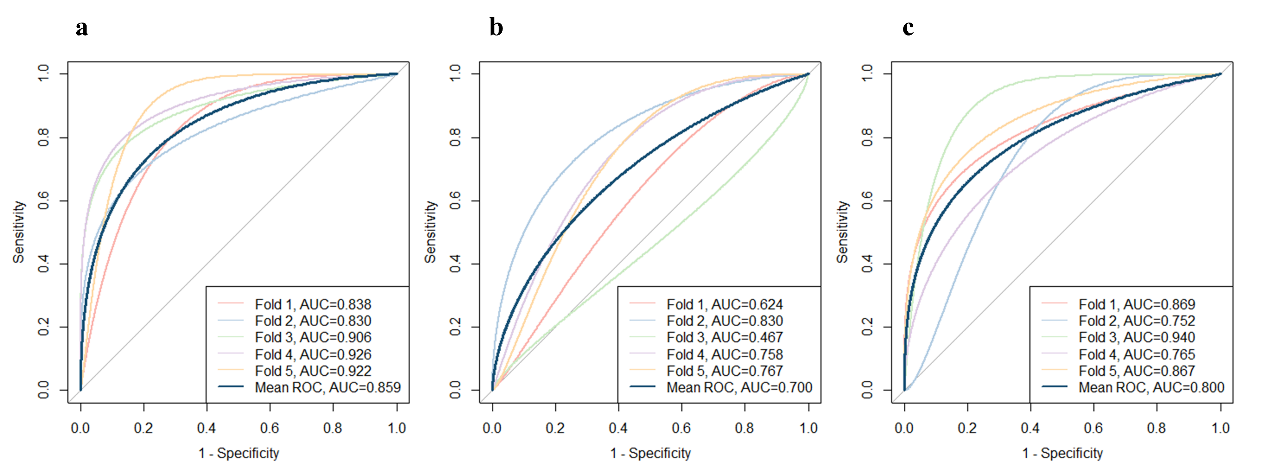
1、Hill NR, Fatoba ST, Oke JL, et al. Global prevalence
of chronic kidney disease - A systematic review and
meta-analysis. PLoS One. 2016, 11(7): e0158765. DOI:
10.1371/journal.pone.0158765.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence
of chronic kidney disease - A systematic review and
meta-analysis. PLoS One. 2016, 11(7): e0158765. DOI:
10.1371/journal.pone.0158765.
2、Glassock RJ, Warnock DG, Delanaye P. The global burden
of chronic kidney disease: estimates, variability and
pitfalls. Nat Rev Nephrol. 2017, 13(2): 104-114. DOI:
10.1038/nrneph.2016.163.Glassock RJ, Warnock DG, Delanaye P. The global burden
of chronic kidney disease: estimates, variability and
pitfalls. Nat Rev Nephrol. 2017, 13(2): 104-114. DOI:
10.1038/nrneph.2016.163.
3、Mills KT, Xu Y, Zhang W, et al. A systematic analysis of
worldwide population-based data on the global burden of
chronic kidney disease in 2010. Kidney Int. 2015, 88(5):
950-957. DOI: 10.1038/ki.2015.230.Mills KT, Xu Y, Zhang W, et al. A systematic analysis of
worldwide population-based data on the global burden of
chronic kidney disease in 2010. Kidney Int. 2015, 88(5):
950-957. DOI: 10.1038/ki.2015.230.
4、Bikbov B, Perico N, Remuzzi G, et al. Disparities in
chronic kidney disease prevalence among males and
females in 195 countries: analysis of the global burden of
disease 2016 study. Nephron. 2018, 139(4): 313-318. DOI:
10.1159/000489897Bikbov B, Perico N, Remuzzi G, et al. Disparities in
chronic kidney disease prevalence among males and
females in 195 countries: analysis of the global burden of
disease 2016 study. Nephron. 2018, 139(4): 313-318. DOI:
10.1159/000489897
5、Querfeld U, Mak RH, Pries AR. Microvascular disease
in chronic kidney disease: the base of the iceberg in
cardiovascular comorbidity. Clin Sci. 2020, 134(12): 1333-
1356. DOI: 10.1042/CS20200279.Querfeld U, Mak RH, Pries AR. Microvascular disease
in chronic kidney disease: the base of the iceberg in
cardiovascular comorbidity. Clin Sci. 2020, 134(12): 1333-
1356. DOI: 10.1042/CS20200279.
6、Jankowski J, Floege J, Fliser D, et al. Cardiovascular
disease in chronic kidney disease: pathophysiological
insights and therapeutic options. Circulation. 2021,
143(11): 1157-1172. DOI: 10.1161/CIRCULATIONAHA.
120.050686.Jankowski J, Floege J, Fliser D, et al. Cardiovascular
disease in chronic kidney disease: pathophysiological
insights and therapeutic options. Circulation. 2021,
143(11): 1157-1172. DOI: 10.1161/CIRCULATIONAHA.
120.050686.
7、Bello AK, Alrukhaimi M, Ashuntantang GE, et al.
Complications of chronic kidney disease: current state,
knowledge gaps, and strategy for action. Kidney Int Suppl.
2017, 7(2): 122-129. DOI: 10.1016/j.kisu.2017.07.007.Bello AK, Alrukhaimi M, Ashuntantang GE, et al.
Complications of chronic kidney disease: current state,
knowledge gaps, and strategy for action. Kidney Int Suppl.
2017, 7(2): 122-129. DOI: 10.1016/j.kisu.2017.07.007.
8、Viggiano D, Wagner CA, Martino G, et al. Mechanisms
of cognitive dysfunction in CKD. Nat Rev Nephrol. 2020,
16(8): 452-469. DOI: 10.1038/s41581-020-0266-9.Viggiano D, Wagner CA, Martino G, et al. Mechanisms
of cognitive dysfunction in CKD. Nat Rev Nephrol. 2020,
16(8): 452-469. DOI: 10.1038/s41581-020-0266-9.
9、Hamed SA. Neurologic conditions and disorders of uremic
syndrome of chronic kidney disease: presentations, causes,
and treatment strategies. Expert Rev Clin Pharmacol. 2019,
12(1): 61-90. DOI: 10.1080/17512433.2019.1555468.Hamed SA. Neurologic conditions and disorders of uremic
syndrome of chronic kidney disease: presentations, causes,
and treatment strategies. Expert Rev Clin Pharmacol. 2019,
12(1): 61-90. DOI: 10.1080/17512433.2019.1555468.
10、Chang J, Ko A, Park SM, et al. Association of
Cardiovascular Mortality and Deep Learning-Funduscopic
Atherosclerosis Score derived from retinal fundus
images. Am J Ophthalmol. 2020, 217: 121-130. DOI: 10.1016/j.ajo.2020.03.027.Chang J, Ko A, Park SM, et al. Association of
Cardiovascular Mortality and Deep Learning-Funduscopic
Atherosclerosis Score derived from retinal fundus
images. Am J Ophthalmol. 2020, 217: 121-130. DOI: 10.1016/j.ajo.2020.03.027.
11、Cheung CYL, Ikram MK, Chen C, et al. Imaging retina to
study dementia and stroke. Prog Retin Eye Res. 2017, 57:
89-107. DOI: 10.1016/j.preteyeres.2017.01.001.Cheung CYL, Ikram MK, Chen C, et al. Imaging retina to
study dementia and stroke. Prog Retin Eye Res. 2017, 57:
89-107. DOI: 10.1016/j.preteyeres.2017.01.001.
12、Xiao W, Huang X, Wang JH, et al. Screening and
identifying hepatobiliary diseases through deep learning
using ocular images: a prospective, multicentre study.
Lancet Digit Health. 2021, 3(2): e88-e97. DOI: 10.1016/
S2589-7500(20)30288-0Xiao W, Huang X, Wang JH, et al. Screening and
identifying hepatobiliary diseases through deep learning
using ocular images: a prospective, multicentre study.
Lancet Digit Health. 2021, 3(2): e88-e97. DOI: 10.1016/
S2589-7500(20)30288-0
13、Wong CW, Wong TY, Cheng CY, et al. Kidney and eye
diseases: common risk factors, etiological mechanisms,
and pathways. Kidney Int. 2014, 85(6): 1290-1302. DOI:
10.1038/ki.2013.491.Wong CW, Wong TY, Cheng CY, et al. Kidney and eye
diseases: common risk factors, etiological mechanisms,
and pathways. Kidney Int. 2014, 85(6): 1290-1302. DOI:
10.1038/ki.2013.491.
14、Zhang K, Liu X, Xu J, et al. Deep-learning models for
the detection and incidence prediction of chronic kidney
disease and type 2 diabetes from retinal fundus images.
Nat Biomed Eng. 2021, 5(6): 533-545. DOI: 10.1038/
s41551-021-00745-6.Zhang K, Liu X, Xu J, et al. Deep-learning models for
the detection and incidence prediction of chronic kidney
disease and type 2 diabetes from retinal fundus images.
Nat Biomed Eng. 2021, 5(6): 533-545. DOI: 10.1038/
s41551-021-00745-6.
15、Huang D, Swanson EA, Lin CP, et al. Optical coherence
tomography. Science. 1991, 254(5035): 1178-1181. DOI:
10.1126/science.1957169.Huang D, Swanson EA, Lin CP, et al. Optical coherence
tomography. Science. 1991, 254(5035): 1178-1181. DOI:
10.1126/science.1957169.
16、Spaide RF, Fujimoto JG, Waheed NK, et al. Optical
coherence tomography angiography. Prog Retin Eye Res.
2018, 64: 1-55. DOI: 10.1016/j.preteyeres.2017.11.003.Spaide RF, Fujimoto JG, Waheed NK, et al. Optical
coherence tomography angiography. Prog Retin Eye Res.
2018, 64: 1-55. DOI: 10.1016/j.preteyeres.2017.11.003.
17、Zeng Y, Cao D, Yu H, et al. Early retinal neurovascular
impairment in patients with diabetes without clinically
detectable retinopathy. Br J Ophthalmol. 2019, 103(12):
1747-1752. DOI: 10.1136/bjophthalmol-2018-313582.Zeng Y, Cao D, Yu H, et al. Early retinal neurovascular
impairment in patients with diabetes without clinically
detectable retinopathy. Br J Ophthalmol. 2019, 103(12):
1747-1752. DOI: 10.1136/bjophthalmol-2018-313582.
18、Gong X, Wang W, Li W, et al. Association between renal
function and retinal neurodegeneration in Chinese patients
with type 2 diabetes mellitus. Ann Transl Med. 2021, 9(7):
560. DOI: 10.21037/atm-20-6957.Gong X, Wang W, Li W, et al. Association between renal
function and retinal neurodegeneration in Chinese patients
with type 2 diabetes mellitus. Ann Transl Med. 2021, 9(7):
560. DOI: 10.21037/atm-20-6957.
19、Zhuang X, Cao D, Zeng Y, et al. Associations between
retinal microvasculature/microstructure and renal function
in type 2 diabetes patients with early chronic kidney
disease. Diabetes Res Clin Pract. 2020, 168: 108373. DOI:
10.1016/j.diabres.2020.108373.Zhuang X, Cao D, Zeng Y, et al. Associations between
retinal microvasculature/microstructure and renal function
in type 2 diabetes patients with early chronic kidney
disease. Diabetes Res Clin Pract. 2020, 168: 108373. DOI:
10.1016/j.diabres.2020.108373.
20、Yeung L, Wu IW, Sun CC, et al. Early retinal
microvascular abnormalities in patients with chronic
kidney disease. Microcirculation. 2019, 26(7): e12555.
DOI: 10.1111/micc.12555.Yeung L, Wu IW, Sun CC, et al. Early retinal
microvascular abnormalities in patients with chronic
kidney disease. Microcirculation. 2019, 26(7): e12555.
DOI: 10.1111/micc.12555.
21、Wang W, He M, Gong X, et al. Association of
r e n a l f u n c t i o n w i t h r e t i n a l v e s s e l d e n s i t y i n
patients with type 2 diabetes by using swept-source
optical coherence tomographic angiography. Br J
Ophthalmol. 2020, 104(12): 1768-1773. DOI: 10.1136/
bjophthalmol-2019-315450.Wang W, He M, Gong X, et al. Association of
r e n a l f u n c t i o n w i t h r e t i n a l v e s s e l d e n s i t y i n
patients with type 2 diabetes by using swept-source
optical coherence tomographic angiography. Br J
Ophthalmol. 2020, 104(12): 1768-1773. DOI: 10.1136/
bjophthalmol-2019-315450.
22、Strain WD, Paldánius PM. Diabetes, cardiovascular
disease and the microcirculation. Cardiovasc Diabetol.
2018, 17(1): 57. DOI: 10.1186/s12933-018-0703-2.Strain WD, Paldánius PM. Diabetes, cardiovascular
disease and the microcirculation. Cardiovasc Diabetol.
2018, 17(1): 57. DOI: 10.1186/s12933-018-0703-2.
23、Zhang B, Chou Y, Zhao X, et al. Early detection of
microvascular impairments with optical coherence
tomography angiography in diabetic patients without
clinical retinopathy: a meta-analysis. Am J Ophthalmol.
2021, 222: 226-237. DOI: 10.1016/j.ajo.2020.09.032.Zhang B, Chou Y, Zhao X, et al. Early detection of
microvascular impairments with optical coherence
tomography angiography in diabetic patients without
clinical retinopathy: a meta-analysis. Am J Ophthalmol.
2021, 222: 226-237. DOI: 10.1016/j.ajo.2020.09.032.
24、Liu L, Gao J, Bao W, et al. Analysis of foveal
microvascular abnormalities in diabetic retinopathy using
optical coherence tomography angiography with projection
artifact removal. J Ophthalmol. 2018, 2018: 3926745.
DOI: 10.1155/2018/3926745.Liu L, Gao J, Bao W, et al. Analysis of foveal
microvascular abnormalities in diabetic retinopathy using
optical coherence tomography angiography with projection
artifact removal. J Ophthalmol. 2018, 2018: 3926745.
DOI: 10.1155/2018/3926745.
25、Manns L, Scott-Douglas N, Tonelli M, et al. A population-based analysis of quality indicators in CKD. Clin J
Am Soc Nephrol. 2017,12(5):727-733. DOI:10.2215/
CJN.08720816.Manns L, Scott-Douglas N, Tonelli M, et al. A population-based analysis of quality indicators in CKD. Clin J
Am Soc Nephrol. 2017,12(5):727-733. DOI:10.2215/
CJN.08720816.
26、Weckmann GFC, Stracke S, Haase A, et al. Diagnosis and
management of non-dialysis chronic kidney disease in
ambulatory care: a systematic review of clinical practice
guidelines. BMC Nephrol. 2018, 19(1): 258. DOI:
10.1186/s12882-018-1048-5.Weckmann GFC, Stracke S, Haase A, et al. Diagnosis and
management of non-dialysis chronic kidney disease in
ambulatory care: a systematic review of clinical practice
guidelines. BMC Nephrol. 2018, 19(1): 258. DOI:
10.1186/s12882-018-1048-5.
27、Anno. Chapter 1: definition and classification of CKD.
Kidney Int Suppl. 2013, 3(1): 19-62. DOI: 10.1038/
kisup.2012.64.Anno. Chapter 1: definition and classification of CKD.
Kidney Int Suppl. 2013, 3(1): 19-62. DOI: 10.1038/
kisup.2012.64.
28、Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med.
2009,150(9):604-612. DOI:10.7326/0003-4819-150-9-
200905050-00006.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med.
2009,150(9):604-612. DOI:10.7326/0003-4819-150-9-
200905050-00006.
29、Wouters OJ, O’Donoghue DJ, Ritchie J, et al. Early
chronic kidney disease: diagnosis, management and
models of care. Nat Rev Nephrol. 2015, 11(8): 491-502.
DOI: 10.1038/nrneph.2015.85.Wouters OJ, O’Donoghue DJ, Ritchie J, et al. Early
chronic kidney disease: diagnosis, management and
models of care. Nat Rev Nephrol. 2015, 11(8): 491-502.
DOI: 10.1038/nrneph.2015.85.
30、Mandrekar JN. Receiver operating characteristic curve in
diagnostic test assessment. J Thorac Oncol. 2010, 5(9):
1315-1316. DOI: 10.1097/JTO.0b013e3181ec173d.Mandrekar JN. Receiver operating characteristic curve in
diagnostic test assessment. J Thorac Oncol. 2010, 5(9):
1315-1316. DOI: 10.1097/JTO.0b013e3181ec173d.
31、Consortium CKDP, Matsushita K, van der Velde M, et
al. Association of estimated glomerular filtration rate and
albuminuria with all-cause and cardiovascular mortality in
general population cohorts: a collaborative meta-analysis.
Lancet. 2010, 375(9731): 2073-2081. DOI: 10.1016/
S0140-6736(10)60674-5.Consortium CKDP, Matsushita K, van der Velde M, et
al. Association of estimated glomerular filtration rate and
albuminuria with all-cause and cardiovascular mortality in
general population cohorts: a collaborative meta-analysis.
Lancet. 2010, 375(9731): 2073-2081. DOI: 10.1016/
S0140-6736(10)60674-5.
32、Monteiro-Henriques I, Rocha-Sousa A, Barbosa-Breda
J. Optical coherence tomography angiography changes
in cardiovascular systemic diseases and risk factors: a
Review. Acta Ophthalmol. 2022, 100(1): e1-e15. DOI:
10.1111/aos.14851.Monteiro-Henriques I, Rocha-Sousa A, Barbosa-Breda
J. Optical coherence tomography angiography changes
in cardiovascular systemic diseases and risk factors: a
Review. Acta Ophthalmol. 2022, 100(1): e1-e15. DOI:
10.1111/aos.14851.
33、Islam MT, Al-Absi HRH, Ruagh EA, et al. DiaNet: a deep
learning based architecture to diagnose diabetes using
retinal images only. IEEE Access. 2021, 9: 15686-15695.
DOI: 10.1109/ACCESS.2021.3052477.Islam MT, Al-Absi HRH, Ruagh EA, et al. DiaNet: a deep
learning based architecture to diagnose diabetes using
retinal images only. IEEE Access. 2021, 9: 15686-15695.
DOI: 10.1109/ACCESS.2021.3052477.
34、Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular
anatomy of the human retina by projection-resolved
optical coherence tomography angiography. Sci Rep.
2017, 7:42201. DOI: 10.1038/srep42201.Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular
anatomy of the human retina by projection-resolved
optical coherence tomography angiography. Sci Rep.
2017, 7:42201. DOI: 10.1038/srep42201.
35、Nian S, Lo ACY, Mi Y, et al. Neurovascular unit in
diabetic retinopathy: pathophysiological roles and
potential therapeutical targets. Eye Vis. 2021, 8(1): 15.
DOI: 10.1186/s40662-021-00239-1.Nian S, Lo ACY, Mi Y, et al. Neurovascular unit in
diabetic retinopathy: pathophysiological roles and
potential therapeutical targets. Eye Vis. 2021, 8(1): 15.
DOI: 10.1186/s40662-021-00239-1.
36、Hawkins BT, Davis TP. The blood-brain barrier/
neurovascular unit in health and disease. Pharmacol Rev.
2005, 57(2): 173-185. DOI: 10.1124/pr.57.2.4.Hawkins BT, Davis TP. The blood-brain barrier/
neurovascular unit in health and disease. Pharmacol Rev.
2005, 57(2): 173-185. DOI: 10.1124/pr.57.2.4.
37、Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy.
N Engl J Med. 2012, 366(13): 1227-1239. DOI: 10.1056/
NEJMra1005073.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy.
N Engl J Med. 2012, 366(13): 1227-1239. DOI: 10.1056/
NEJMra1005073.
38、Tham YC, Cheng CY, Zheng Y, et al. Relationship between
retinal vascular geometry with retinal nerve fiber layer and
ganglion cell-inner plexiform layer in nonglaucomatous
eyes. Invest Ophthalmol Vis Sci. 2013, 54(12): 7309-7316.
DOI: 10.1167/iovs.13-12796.Tham YC, Cheng CY, Zheng Y, et al. Relationship between
retinal vascular geometry with retinal nerve fiber layer and
ganglion cell-inner plexiform layer in nonglaucomatous
eyes. Invest Ophthalmol Vis Sci. 2013, 54(12): 7309-7316.
DOI: 10.1167/iovs.13-12796.
39、Yu DY, Cringle SJ, Yu PK, et al. Retinal capillary
perfusion: spatial and temporal heterogeneity. Prog
Retin Eye Res. 2019, 70: 23-54. DOI: 10.1016/
j.preteyeres.2019.01.001.Yu DY, Cringle SJ, Yu PK, et al. Retinal capillary
perfusion: spatial and temporal heterogeneity. Prog
Retin Eye Res. 2019, 70: 23-54. DOI: 10.1016/
j.preteyeres.2019.01.001.
40、Nusinovici S, Sabanayagam C, Teo BW, et al. Vision
impairment in CKD patients: epidemiology, mechanisms,
differential diagnoses, and prevention. Am J Kidney Dis.
2019, 73(6): 846-857. DOI: 10.1053/j.ajkd.2018.12.047Nusinovici S, Sabanayagam C, Teo BW, et al. Vision
impairment in CKD patients: epidemiology, mechanisms,
differential diagnoses, and prevention. Am J Kidney Dis.
2019, 73(6): 846-857. DOI: 10.1053/j.ajkd.2018.12.047
41、Winkelmayer WC, Eigner M, Berger O, et al. Optic
neuropathy in uremia: an interdisciplinary emergency.
Am J Kidney Dis. 2001, 37(3): E23. DOI: 10.1053/
ajkd.2001.22101.Winkelmayer WC, Eigner M, Berger O, et al. Optic
neuropathy in uremia: an interdisciplinary emergency.
Am J Kidney Dis. 2001, 37(3): E23. DOI: 10.1053/
ajkd.2001.22101.
42、Lamirel C, Newman N, Biousse V. The use of optical
coherence tomography in neurology. Rev Neurol Dis.
2009, 6(4): E105-E120.Lamirel C, Newman N, Biousse V. The use of optical
coherence tomography in neurology. Rev Neurol Dis.
2009, 6(4): E105-E120.
43、She X, Guo J, Liu X, et al. Reliability of vessel density
measurements in the peripapillary retina and correlation
with retinal nerve fiber layer thickness in healthy subjects
using optical coherence tomography angiography.
Ophthalmologica. 2018, 240(4): 183-190. DOI:
10.1159/000485957.She X, Guo J, Liu X, et al. Reliability of vessel density
measurements in the peripapillary retina and correlation
with retinal nerve fiber layer thickness in healthy subjects
using optical coherence tomography angiography.
Ophthalmologica. 2018, 240(4): 183-190. DOI:
10.1159/000485957.
44、Nesper PL, Fawzi AA. Human parafoveal capillary
vascular anatomy and connectivity revealed by optical
coherence tomography angiography. Invest Ophthalmol
Vis Sci. 2018, 59(10): 3858-3867. DOI: 10.1167/iovs.18-
24710.Nesper PL, Fawzi AA. Human parafoveal capillary
vascular anatomy and connectivity revealed by optical
coherence tomography angiography. Invest Ophthalmol
Vis Sci. 2018, 59(10): 3858-3867. DOI: 10.1167/iovs.18-
24710.
45、Kohan DE, Barton M. Endothelin and endothelin
antagonists in chronic kidney disease. Kidney Int. 2014, 86(5): 896-904. DOI: 10.1038/ki.2014.143.Kohan DE, Barton M. Endothelin and endothelin
antagonists in chronic kidney disease. Kidney Int. 2014, 86(5): 896-904. DOI: 10.1038/ki.2014.143.
46、Ehling%20J%2C%20B%C3%A1b%C3%AD%C4%8Dkov%C3%A1%20J%2C%20Gremse%20F%2C%20et%20al.%20Quantitative%20micro%02computed%20tomography%20imaging%20of%20vascular%20dysfunction%20%0Ain%20progressive%20kidney%20diseases.%20J%20Am%20Soc%20Nephrol.%202016%2C%20%0A27(2)%3A%20520-532.%20DOI%3A%2010.1681%2FASN.2015020204.Ehling%20J%2C%20B%C3%A1b%C3%AD%C4%8Dkov%C3%A1%20J%2C%20Gremse%20F%2C%20et%20al.%20Quantitative%20micro%02computed%20tomography%20imaging%20of%20vascular%20dysfunction%20%0Ain%20progressive%20kidney%20diseases.%20J%20Am%20Soc%20Nephrol.%202016%2C%20%0A27(2)%3A%20520-532.%20DOI%3A%2010.1681%2FASN.2015020204.
47、Culshaw GJ, MacIntyre IM, Dhaun N, et al. Endothelin in
nondiabetic chronic kidney disease: preclinical and clinical
studies. Semin Nephrol. 2015, 35(2): 176-187. DOI:
10.1016/j.semnephrol.2015.03.002.Culshaw GJ, MacIntyre IM, Dhaun N, et al. Endothelin in
nondiabetic chronic kidney disease: preclinical and clinical
studies. Semin Nephrol. 2015, 35(2): 176-187. DOI:
10.1016/j.semnephrol.2015.03.002.
48、Dhaun N, MacIntyre IM, Melville V, et al. Blood pressure�independent reduction in proteinuria and arterial stiffness
after acute endothelin-a receptor antagonism in chronic
kidney disease. Hypertension. 2009, 54(1): 113-119. DOI:
10.1161/HYPERTENSIONAHA.109.132670.Dhaun N, MacIntyre IM, Melville V, et al. Blood pressure�independent reduction in proteinuria and arterial stiffness
after acute endothelin-a receptor antagonism in chronic
kidney disease. Hypertension. 2009, 54(1): 113-119. DOI:
10.1161/HYPERTENSIONAHA.109.132670.
49、Dhaun N, Moorhouse R, MacIntyre IM, et al. Diurnal
variation in blood pressure and arterial stiffness in
chronic kidney disease: the role of endothelin-1.
Hypertension. 2014, 64(2): 296-304. DOI: 10.1161/
HYPERTENSIONAHA.114.03533.Dhaun N, Moorhouse R, MacIntyre IM, et al. Diurnal
variation in blood pressure and arterial stiffness in
chronic kidney disease: the role of endothelin-1.
Hypertension. 2014, 64(2): 296-304. DOI: 10.1161/
HYPERTENSIONAHA.114.03533.
50、Moir J, Khanna S, Skondra D. Review of OCT
angiography findings in diabetic retinopathy: insights and
perspectives. Int J Transl Med. 2021, 1(3): 286-305. DOI:
10.3390/ijtm1030017.Moir J, Khanna S, Skondra D. Review of OCT
angiography findings in diabetic retinopathy: insights and
perspectives. Int J Transl Med. 2021, 1(3): 286-305. DOI:
10.3390/ijtm1030017.
51、Takase N, Nozaki M, Kato A, et al. Enlargement of foveal
avascular zone in diabetic eyes evaluated by en face optical
coherence tomography angiography. Retina. 2015, 35(11):
2377-2383. DOI: 10.1097/IAE.0000000000000849.Takase N, Nozaki M, Kato A, et al. Enlargement of foveal
avascular zone in diabetic eyes evaluated by en face optical
coherence tomography angiography. Retina. 2015, 35(11):
2377-2383. DOI: 10.1097/IAE.0000000000000849.
52、Kasumovic A, Matoc I, Rebic D, et al. Assessment
of retinal microangiopathy in chronic kidney disease
patients. Med Arch. 2020, 74(3): 191-194. DOI: 10.5455/
medarh.2020.74.191-194.Kasumovic A, Matoc I, Rebic D, et al. Assessment
of retinal microangiopathy in chronic kidney disease
patients. Med Arch. 2020, 74(3): 191-194. DOI: 10.5455/
medarh.2020.74.191-194.
53、Rosen RB, Andrade Romo JS, Krawitz BD, et al. Earliest
evidence of preclinical diabetic retinopathy revealed using
optical coherence tomography angiography perfused
capillary density. Am J Ophthalmol. 2019, 203: 103-115.
DOI: 10.1016/j.ajo.2019.01.012.Rosen RB, Andrade Romo JS, Krawitz BD, et al. Earliest
evidence of preclinical diabetic retinopathy revealed using
optical coherence tomography angiography perfused
capillary density. Am J Ophthalmol. 2019, 203: 103-115.
DOI: 10.1016/j.ajo.2019.01.012.