1、Shields CL, Alset AE, Boal NS, et al. Conjunctival tumors in 5002 cases. comparative analysis of benign versus malignant counterparts. the 2016 James D. Allen lecture. Am J Ophthalmol. 2017, 173: 106-133. DOI: 10.1016/j.ajo.2016.09.034.Shields CL, Alset AE, Boal NS, et al. Conjunctival tumors in 5002 cases. comparative analysis of benign versus malignant counterparts. the 2016 James D. Allen lecture. Am J Ophthalmol. 2017, 173: 106-133. DOI: 10.1016/j.ajo.2016.09.034.
2、Shields CL, Demirci H, Karatza E, et al. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004, 111(9): 1747-1754. DOI: 10.1016/j.ophtha.2004.02.013. Shields CL, Demirci H, Karatza E, et al. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004, 111(9): 1747-1754. DOI: 10.1016/j.ophtha.2004.02.013.
3、Maheshwari A, Finger PT. Cancers of the eye. Cancer Metastasis Rev. 2018, 37(4): 677-690. DOI: 10.1007/s10555-018-9762-9. Maheshwari A, Finger PT. Cancers of the eye. Cancer Metastasis Rev. 2018, 37(4): 677-690. DOI: 10.1007/s10555-018-9762-9.
4、Henriksen JR, Ramberg I, Mikkelsen LH, et al. The role of infectious agents in cancer of the ocular region[J]. Apmis, 2020, 128(2): 136-149. DOI: 10.1111/apm.13017. Henriksen JR, Ramberg I, Mikkelsen LH, et al. The role of infectious agents in cancer of the ocular region[J]. Apmis, 2020, 128(2): 136-149. DOI: 10.1111/apm.13017.
5、Verma V, Shen D, Sieving PC, et al. The role of infectious agents in the etiology of ocular adnexal neoplasia. Surv Ophthalmol. 2008, 53(4): 312-331. DOI: 10.1016/j.survophthal.2008.04.008.Verma V, Shen D, Sieving PC, et al. The role of infectious agents in the etiology of ocular adnexal neoplasia. Surv Ophthalmol. 2008, 53(4): 312-331. DOI: 10.1016/j.survophthal.2008.04.008.
6、Auw-H%C3%A4drich%20C%2C%20G%C3%B6bel%20N%2C%20Illerhaus%20G.%20Infectious%20agents%20in%20ocular%20adnexal%20tumours.%20Klin%20Monbl%20Augenheilkd.%202010%2C%20227(7)%3A%20530-537.%20DOI%3A%2010.1055%2Fs-0029-1245153.%20Auw-H%C3%A4drich%20C%2C%20G%C3%B6bel%20N%2C%20Illerhaus%20G.%20Infectious%20agents%20in%20ocular%20adnexal%20tumours.%20Klin%20Monbl%20Augenheilkd.%202010%2C%20227(7)%3A%20530-537.%20DOI%3A%2010.1055%2Fs-0029-1245153.%20
7、Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol. 2004, 49(1): 3-24. DOI: 10.1016/j.survophthal.2003.10.008. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol. 2004, 49(1): 3-24. DOI: 10.1016/j.survophthal.2003.10.008.
8、Shields CL, Ramasubramanian A, Mellen PL, et al. Conjunctival squamous cell carcinoma arising in immunosuppressed patients (organ transplant, human immunodeficiency virus infection). Ophthalmology. 2011, 118(11): 2133-2137. DOI: 10.1016/j.ophtha.2011.04.001.Shields CL, Ramasubramanian A, Mellen PL, et al. Conjunctival squamous cell carcinoma arising in immunosuppressed patients (organ transplant, human immunodeficiency virus infection). Ophthalmology. 2011, 118(11): 2133-2137. DOI: 10.1016/j.ophtha.2011.04.001.
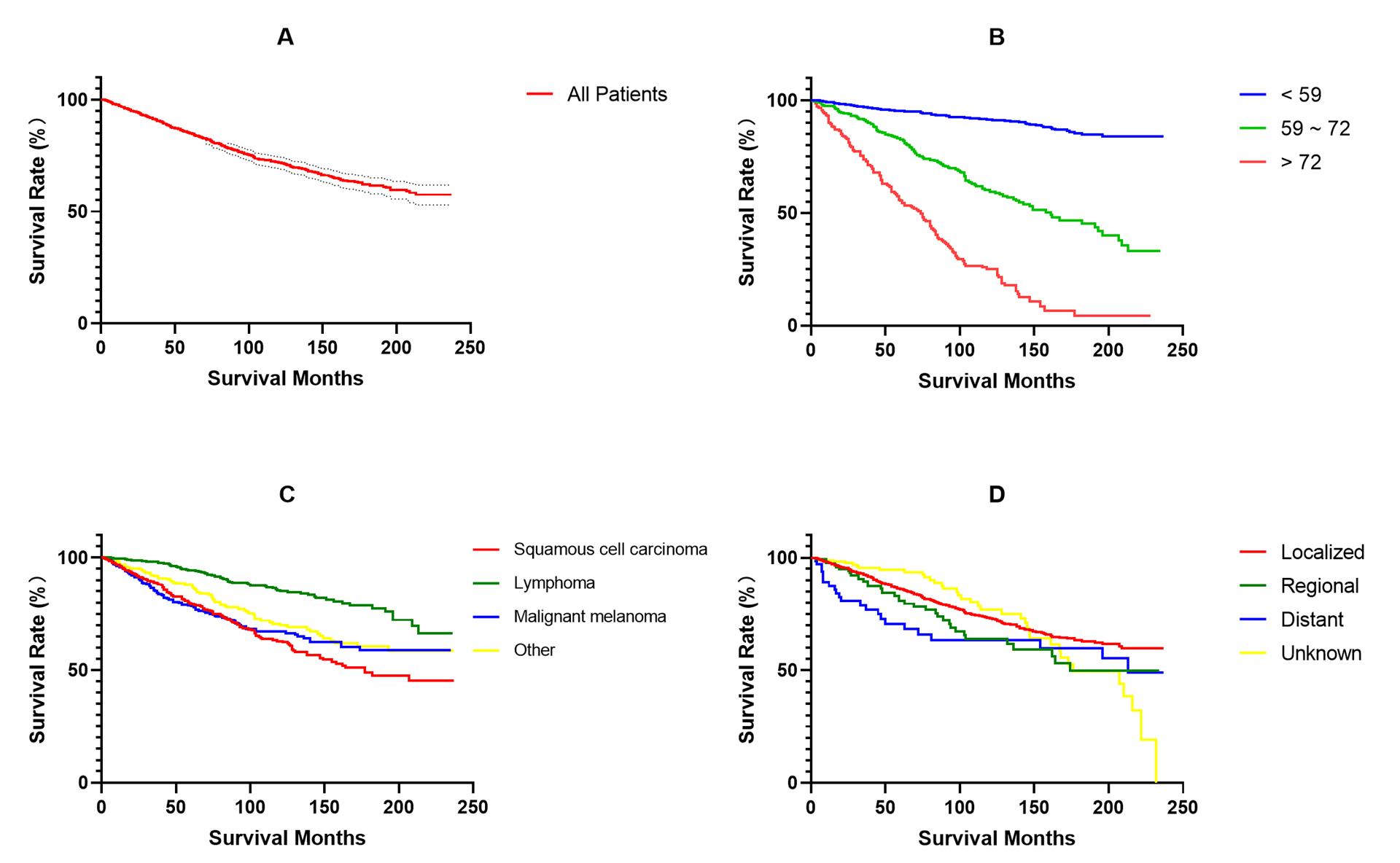
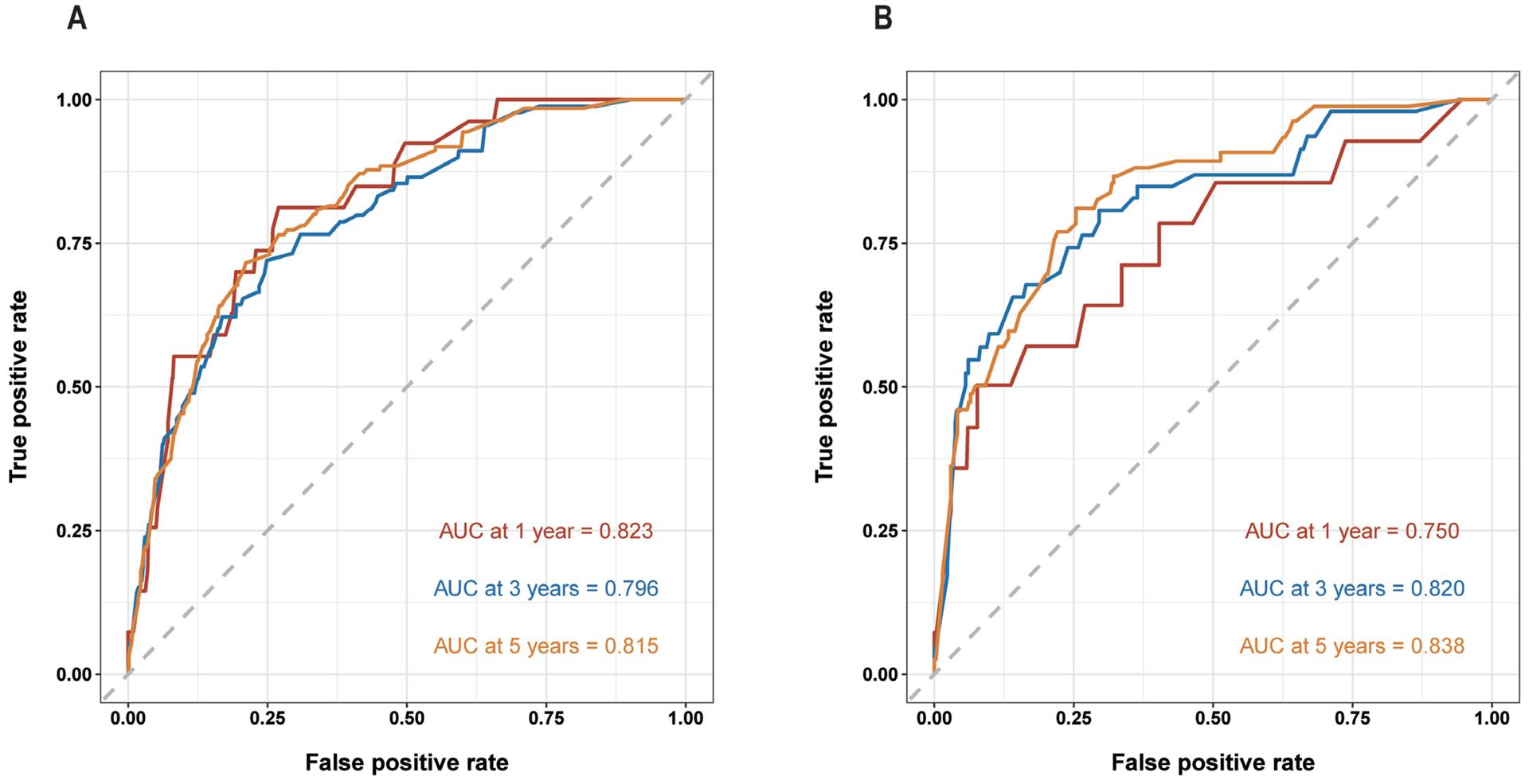
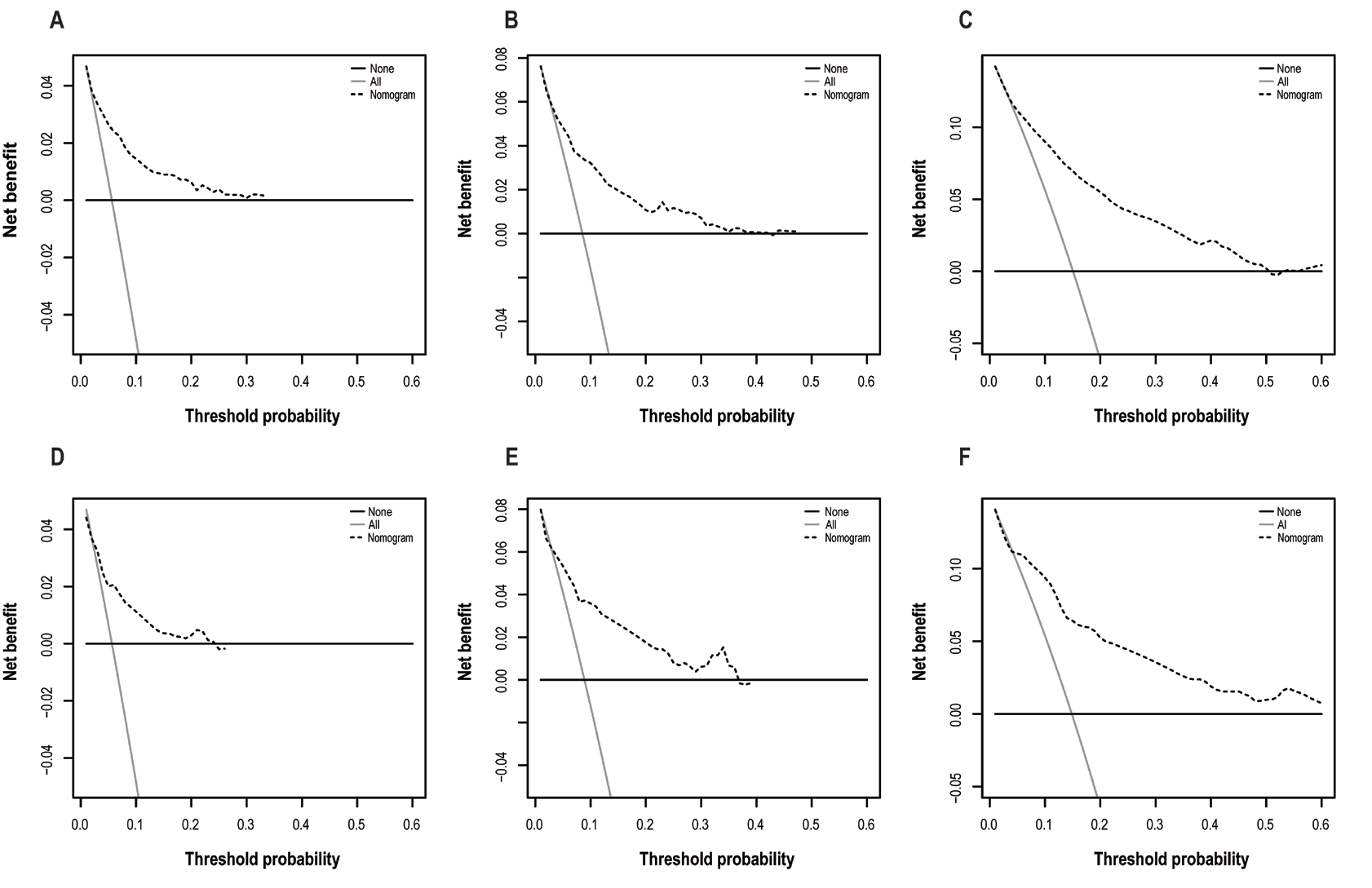
9、Diao Y, Li X, Huo Y, et al. Epidemiological analysis and prognosis of conjunctival cancer in the past twenty years: a population-based retrospective study using SEER data. Biomed Res Int. 2020, 2020: 1203938. DOI: 10.1155/2020/1203938. Diao Y, Li X, Huo Y, et al. Epidemiological analysis and prognosis of conjunctival cancer in the past twenty years: a population-based retrospective study using SEER data. Biomed Res Int. 2020, 2020: 1203938. DOI: 10.1155/2020/1203938.
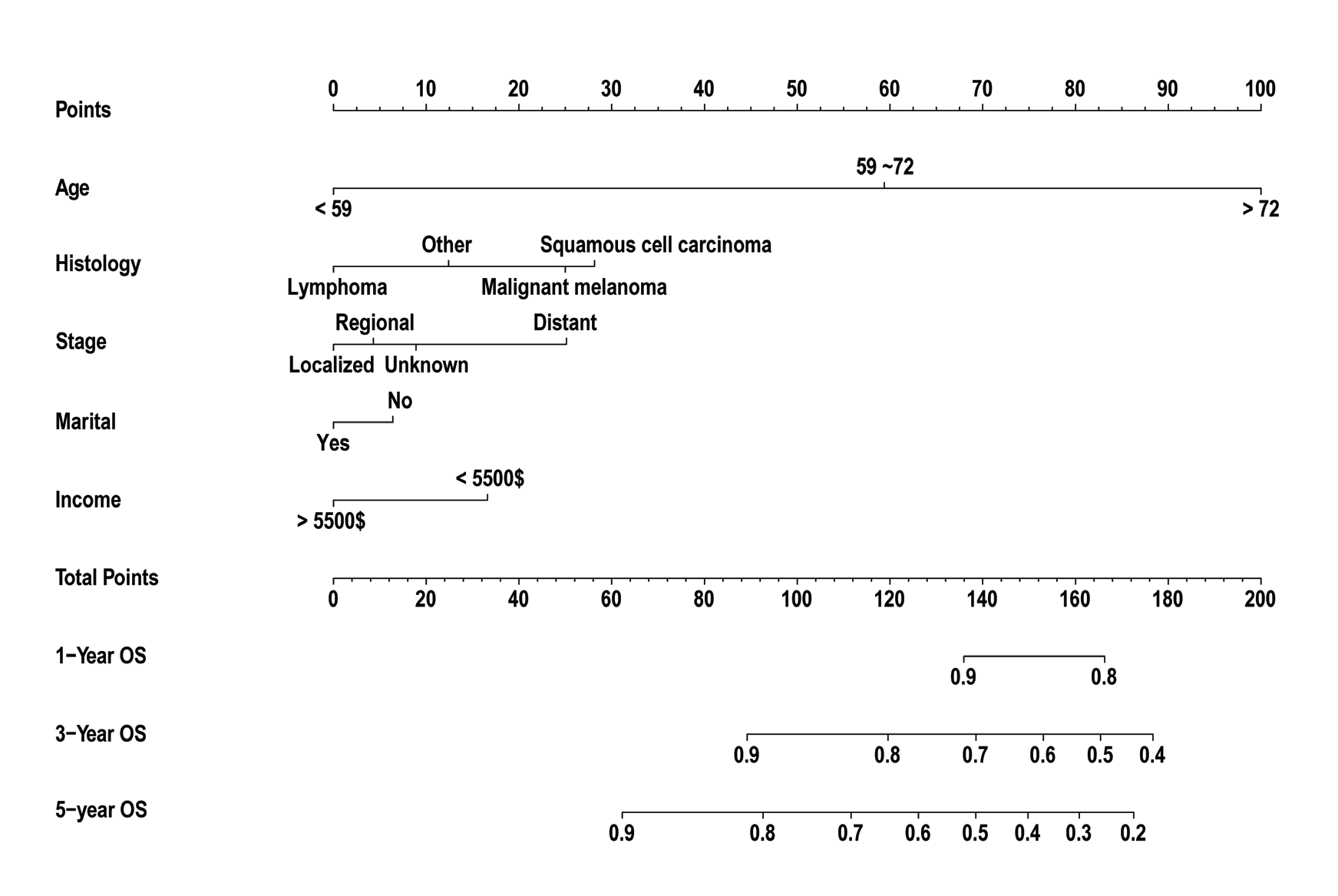
10、Abt NB, Zhao J, Huang Y, et al. Prognostic factors and survival for malignant conjunctival melanoma and squamous cell carcinoma over four decades. Am J Otolaryngol. 2019, 40(4): 577-582. DOI: 10.1016/j.amjoto.2019.05.013.Abt NB, Zhao J, Huang Y, et al. Prognostic factors and survival for malignant conjunctival melanoma and squamous cell carcinoma over four decades. Am J Otolaryngol. 2019, 40(4): 577-582. DOI: 10.1016/j.amjoto.2019.05.013.
11、Kirkegaard MM, Rasmussen PK, et al. Conjunctival Lymphoma-An International Multicenter Retrospective Study. JAMA Ophthalmol. 2016, 134(4): 406-14. DOI: 10.1001/jamaophthalmol.2015.6122.Kirkegaard MM, Rasmussen PK, et al. Conjunctival Lymphoma-An International Multicenter Retrospective Study. JAMA Ophthalmol. 2016, 134(4): 406-14. DOI: 10.1001/jamaophthalmol.2015.6122.
12、Sun EC, Fears TR, Goedert JJ. Epidemiology of squamous cell conjunctival cancer. Cancer Epidemiol Biomarkers Prev. 1997, 6(2): 73-77.Sun EC, Fears TR, Goedert JJ. Epidemiology of squamous cell conjunctival cancer. Cancer Epidemiol Biomarkers Prev. 1997, 6(2): 73-77.
13、Shields CL, Chien JL, Surakiatchanukul T, et al. Conjunctival tumors: review of clinical features, risks, biomarkers, and outcomes: the 2017 J. donald M. gass lecture. Asia Pac J Ophthalmol (Phila). 2017, 6(2): 109-120. DOI: 10.22608/APO.201710. Shields CL, Chien JL, Surakiatchanukul T, et al. Conjunctival tumors: review of clinical features, risks, biomarkers, and outcomes: the 2017 J. donald M. gass lecture. Asia Pac J Ophthalmol (Phila). 2017, 6(2): 109-120. DOI: 10.22608/APO.201710.
14、Hu DN, Yu G, McCormick SA, et al. Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanomas. Am J Ophthalmol. 2008, 145(3): 418-423. DOI: 10.1016/j.ajo.2007.10.022. Hu DN, Yu G, McCormick SA, et al. Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanomas. Am J Ophthalmol. 2008, 145(3): 418-423. DOI: 10.1016/j.ajo.2007.10.022.
15、Dalvin%20LA%2C%20Salom%C3%A3o%20DR%2C%20Patel%20SV.%20Population-based%20incidence%20of%20conjunctival%20tumours%20in%20Olmsted%20County%2C%20Minnesota.%20Br%20J%20Ophthalmol.%202018%2C%20102(12)%3A%201728-1734.%20DOI%3A%2010.1136%2Fbjophthalmol-2017-311530.Dalvin%20LA%2C%20Salom%C3%A3o%20DR%2C%20Patel%20SV.%20Population-based%20incidence%20of%20conjunctival%20tumours%20in%20Olmsted%20County%2C%20Minnesota.%20Br%20J%20Ophthalmol.%202018%2C%20102(12)%3A%201728-1734.%20DOI%3A%2010.1136%2Fbjophthalmol-2017-311530.
16、Yu%20GP%2C%20Hu%20DN%2C%20McCormick%20S%2C%20et%20al.%20Conjunctival%20melanoma%3A%20is%20it%20increasing%20in%20the%20United%20States%3F.%20Am%20J%20Ophthalmol.%202003%2C%20135(6)%3A%20800-806.%20DOI%3A%2010.1016%2Fs0002-9394(02)02288-2.Yu%20GP%2C%20Hu%20DN%2C%20McCormick%20S%2C%20et%20al.%20Conjunctival%20melanoma%3A%20is%20it%20increasing%20in%20the%20United%20States%3F.%20Am%20J%20Ophthalmol.%202003%2C%20135(6)%3A%20800-806.%20DOI%3A%2010.1016%2Fs0002-9394(02)02288-2.
17、Gichuhi S, Sagoo MS, Weiss HA, et al. Epidemiology of ocular surface squamous neoplasia in Africa. Trop Med Int Health. 2013, 18(12): 1424-1443. DOI: 10.1111/tmi.12203. Gichuhi S, Sagoo MS, Weiss HA, et al. Epidemiology of ocular surface squamous neoplasia in Africa. Trop Med Int Health. 2013, 18(12): 1424-1443. DOI: 10.1111/tmi.12203.
18、Triay E, Bergman L, Nilsson B, et al. Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol. 2009, 93(11): 1524-1528. DOI: 10.1136/bjo.2009.157933.Triay E, Bergman L, Nilsson B, et al. Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol. 2009, 93(11): 1524-1528. DOI: 10.1136/bjo.2009.157933.
19、Darwich R, Ghazawi FM, Le M, et al. Epidemiology of invasive ocular surface squamous neoplasia in Canada during 1992-2010. Br J Ophthalmol. 2020, 104(10): 1368-1372. DOI: 10.1136/bjophthalmol-2019-314650.Darwich R, Ghazawi FM, Le M, et al. Epidemiology of invasive ocular surface squamous neoplasia in Canada during 1992-2010. Br J Ophthalmol. 2020, 104(10): 1368-1372. DOI: 10.1136/bjophthalmol-2019-314650.
20、Cruzado-Sanchez D, Tellez WA, Villarreal-Aguilar B, et al. Conjunctival squamous cell carcinoma: prognostic factors for the recurrence and metastasis and clinicopathological characteristics at an oncological hospital in Peru. Br J Ophthalmol. 2020, 104(7): 1010-1015. DOI: 10.1136/bjophthalmol-2019-314058. Cruzado-Sanchez D, Tellez WA, Villarreal-Aguilar B, et al. Conjunctival squamous cell carcinoma: prognostic factors for the recurrence and metastasis and clinicopathological characteristics at an oncological hospital in Peru. Br J Ophthalmol. 2020, 104(7): 1010-1015. DOI: 10.1136/bjophthalmol-2019-314058.
21、Chauhan S, Sen S, Sharma A, et al. American Joint Committee on Cancer Staging and clinicopathological high-risk predictors of ocular surface squamous neoplasia: a study from a tertiary eye center in India. Arch Pathol Lab Med. 2014, 138(11): 1488-1494. DOI: 10.5858/arpa.2013-0353-OA. Chauhan S, Sen S, Sharma A, et al. American Joint Committee on Cancer Staging and clinicopathological high-risk predictors of ocular surface squamous neoplasia: a study from a tertiary eye center in India. Arch Pathol Lab Med. 2014, 138(11): 1488-1494. DOI: 10.5858/arpa.2013-0353-OA.
22、Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000, 98: 471-492.Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000, 98: 471-492.
23、Shields CL, Markowitz JS, Belinsky I, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011, 118(2): 389-395.e1-2. DOI: 10.1016/j.ophtha.2010.06.021. Shields CL, Markowitz JS, Belinsky I, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011, 118(2): 389-395.e1-2. DOI: 10.1016/j.ophtha.2010.06.021.
24、Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994, 78(7): 520-528. DOI: 10.1136/bjo.78.7.520. Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994, 78(7): 520-528. DOI: 10.1136/bjo.78.7.520.
25、Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013, 31(31): 3869-76. DOI: 10.1200/JCO.2013.49.6489.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013, 31(31): 3869-76. DOI: 10.1200/JCO.2013.49.6489.