
It is reported that diclofenac sodium can inhibit fibroblast cells [3] , but the effect of diclofenac sodium on epithelial cells is unclear. By culturing corneal epithelial cells in vitro, the study aims to observe the effect of diclofenac sodium on corneal epithelial cells and to explore the pharmacological mechanism
Diclofenac sodium (Wuhan Wujing Pharmaceutical Factory); Dulbecco's Modified Eagle's Medium/Ham's Nutrient Mixture F-12 (DMEM/F12) (Hyclone, U.S.A.); Fetal calf serum (Hangzhou Sijiqing Biological Engineering Company); TACS Calibur Flow Cytometer (Becton-Dickinson Company, USA); New Zealand white rabbits weighing 1.8 - 2.5 kg (Experimental Animal Center of Chongqing Medical University).
Healthy New Zealand white rabbits were killed and the corneas were removed. The endothelial surface was wiped off, and the epithelial surface was scored gently with a 5# needle. The corneas were cut into 4-6 pieces and then put into a 25 cm² tissue culture flask, which was cultured in a 37℃ incubator with 5% CO2. The culture media was replaced 2 or 3 times a week. Six hours later, the cells began to emigrate from the tissue. The epithelial cells grew to a confluent monolayer about 8 days later, and it was the right time to passage.
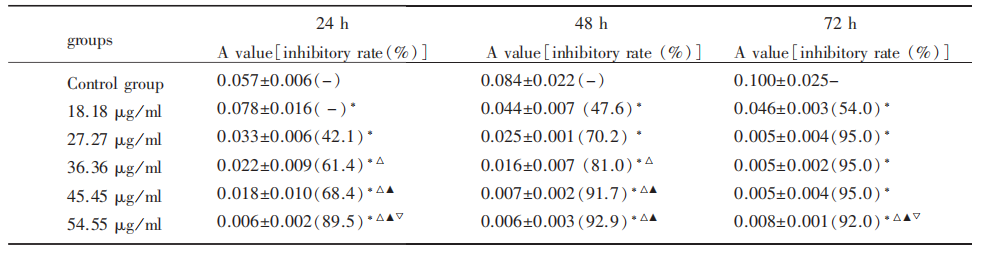
The RCECs of passage 2 were divided into six groups: different concentrations of diclofenac sodium groups and a control group. RCECs in diclofenac sodium groups were incubated with DMEM/F12 containing 18.18, 27.27, 36.36, 45.45, 54.55 μg / ml diclofenac sodium, respectively. The cell proliferation was measured with methyl thiazolyl tetrazolium (MTT) assay 24, 48, and 72 h after incubation, and absorbance A value was read. The inhibition rate was calculated according to the formula: inhibition rate = 1 − average absorbance A value of experimental group average absorbance A value of control group × 100%. IC50 was calculated based on regression equation.
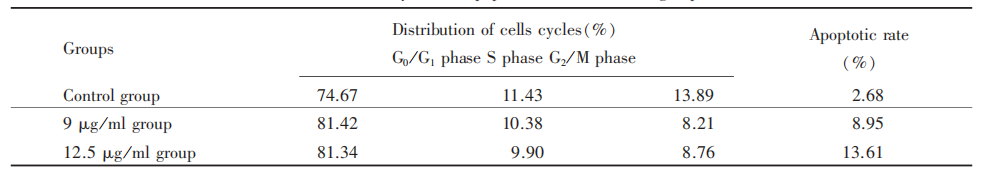
The RCECs were divided into 3 groups: two experimental groups and a control group. The cells in experimental groups were incubated with DMEM/F12 containing 9 and 12.5 μg/ml diclofenac sodium, respectively, while the cells in the control group were cultured with DMEM / F12 only. The cell cycle and apoptotic rate were analyzed by flow cytometer 48h later.
All data were expressed as Mean±SD and analyzed with SPSS 10.0 software. The differences among groups were tested by ANOVA or χ² test, and the pairwise comparisons were tested by Least-significant-difference (LSD). It was considered significant if P<0.05.
MTT assay showed that diclofenac sodium had an obvious inhibitory effect on RCECs. At 24 and 48h, A value descended obviously with the increasing concentrations of diclofenac sodium. The inhibition rate was increasing with the expansion of incubation time in 18.18, 27.27, 36.36, 45.45 μg / ml groups (Tab.1).
There were few apoptotic cells in the control group and obvious apoptotic cells in the experimental groups. After the incubation of diclofenac sodium, the cells in G0/G1 phase increased, while in S phase decreased, and there was a significant difference among the three groups (P<0.01). The apoptotic rates were significantly different among three groups (P<0.01), indicating that the apoptosis cusp and apoptotic rate increased along with the increasing concentration of diclofenac sodium (Tab. 2).
Diclofenac sodium is a kind of non-steroidal anti-inflammatory drugs (NSAIDs), and it can decrease prostaglandin (PG) levels by inhibiting COX to allay fever and ease pain. It has been found that PG can hasten the proliferation of cells, so NSAIDs may exert anti-proliferation effects by decreasing PG levels. Currently, there are many researches on using NSAIDs to prevent and treat tumors [4-5] . Using NSAIDs to suppress neovascularization and prevent posterior capsule opacification (PCO) has also been researched [6-8] . Some investigations revealed that NSAIDs can inhibit the proliferation of cancer cells by inducing apoptosis and ceasing cell cycles [9-10] . Our investigation indicated that diclofenac sodium has an obvious inhibitory effect on RCECs and showed a dosage-effect relation in vitro. The anti-proliferative effect was very apparent on the second day, and the inhibitory rates were all above 90% when the final concentration of diclofenac sodium was above 27.27 μg / ml on the third day. These data indicated that the toxicity of diclofenac sodium increased rapidly along with the extension of the action time. It is also reported from ophthalmological clinics that diclofenac sodium could induce corneal melting. In our investigation, the concentrations of diclofenac sodium were lower than that in the clinic, but the anti-proliferative effect was very apparent. We think the reason may be that the vigor of the corneal epithelial cells in vitro is weaker than in vivo, and tears dilute the eye drops in vivo. So further studies are required to find the best concentration and acting time of diclofenac sodium.
In this study, flow cytometer was used to observe the effect of diclofenac sodium on inducing apoptosis in corneal epithelial cells in order to discuss the mechanism of its anti-proliferative effect. The observation revealed that diclofenac sodium can induce apoptosis in corneal epithelial cells, and the apoptotic rate increased along with the drug's concentration increase. The results also showed that diclofenac sodium changed the cell cycles and increased the cell numbers in G0/G1 phase. The apoptotic rates in the experimental groups were higher than that in the control group, and typical apoptosis cusp appeared in the experimental groups.
In summary, the study proved that diclofenac sodium had an obvious inhibitory effect on RCECs, and the possible mechanisms of its anti-proliferative effect were inducing cell apoptosis and ceasing cell cycles. So it provided the experimental evidence for using diclofenac sodium to inhibit epithelial ingrowth after LASIK.


点击右上角菜单,浏览器打开下载